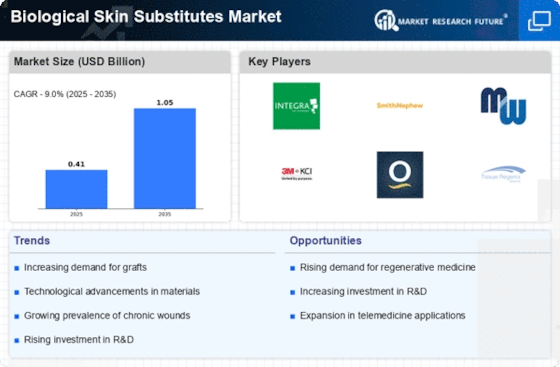
Rising Incidence of Chronic Wounds
The increasing prevalence of chronic wounds, such as diabetic ulcers and pressure sores, is a primary driver for the Biological Skin Substitutes Market. Chronic wounds affect millions of individuals worldwide, leading to significant healthcare costs and a demand for effective treatment options. According to recent estimates, chronic wounds affect approximately 6.5 million patients annually, creating a substantial market opportunity for biological skin substitutes Market. These products offer advanced healing properties, which are essential for managing complex wound care. As the population ages and the incidence of diabetes rises, the need for innovative solutions in wound management becomes more pressing. This trend is likely to propel the growth of the Biological Skin Substitutes Market, as healthcare providers seek to improve patient outcomes and reduce the burden of chronic wounds.
Advancements in Regenerative Medicine
The field of regenerative medicine is rapidly evolving, significantly impacting the Biological Skin Substitutes Market. Innovations in tissue engineering and stem cell research are paving the way for the development of advanced biological skin substitutes Market that can mimic natural skin properties. These advancements not only enhance the efficacy of wound healing but also reduce the risk of complications associated with traditional treatments. The market for regenerative medicine is projected to reach USD 63.5 billion by 2025, indicating a robust growth trajectory. As healthcare professionals increasingly adopt these cutting-edge technologies, the demand for biological skin substitutes Market is expected to rise, further driving the market forward. This trend highlights the potential for biological skin substitutes Market to play a crucial role in the future of wound care and tissue regeneration.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supporting the development and approval of innovative therapies, which is beneficial for the Biological Skin Substitutes Market. Streamlined approval processes and favorable regulations are encouraging manufacturers to invest in research and development of new biological skin substitutes Market. This regulatory support is crucial for bringing advanced products to market more efficiently, thereby meeting the growing demand for effective wound care solutions. Recent initiatives by regulatory agencies have focused on expediting the review of regenerative medicine products, which could lead to a surge in new offerings within the Biological Skin Substitutes Market. As these innovative therapies gain traction, the market is expected to expand, driven by the introduction of novel products that address unmet clinical needs.
Growing Awareness of Advanced Wound Care Solutions
There is a notable increase in awareness regarding advanced wound care solutions among healthcare professionals and patients, which is positively influencing the Biological Skin Substitutes Market. Educational initiatives and training programs are being implemented to inform stakeholders about the benefits of biological skin substitutes Market. This heightened awareness is leading to a shift in treatment paradigms, with more healthcare providers opting for advanced solutions over traditional methods. Market data suggests that the advanced wound care market is expected to grow at a CAGR of 6.5% from 2023 to 2028, reflecting the increasing preference for innovative products. As awareness continues to spread, the Biological Skin Substitutes Market is likely to experience accelerated growth, driven by the demand for effective and efficient wound management solutions.
Increasing Investment in Healthcare Infrastructure
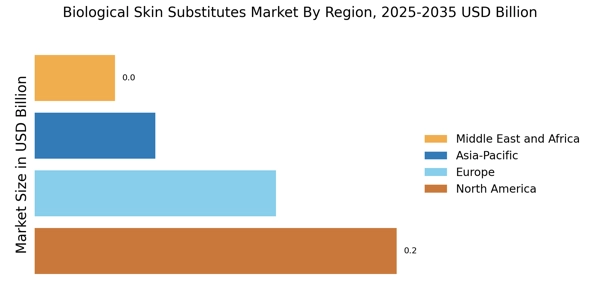
The ongoing investment in healthcare infrastructure across various regions is a significant driver for the Biological Skin Substitutes Market. Governments and private entities are allocating substantial resources to enhance healthcare facilities, particularly in developing regions. This investment is aimed at improving access to advanced medical technologies, including biological skin substitutes Market. As healthcare systems evolve, the demand for innovative wound care solutions is expected to rise. For instance, the healthcare expenditure in several countries is projected to increase by 5% annually, creating a favorable environment for the adoption of biological skin substitutes Market. This trend indicates that as healthcare infrastructure improves, the Biological Skin Substitutes Market will likely benefit from increased accessibility and availability of advanced treatment options.