Technological Innovations
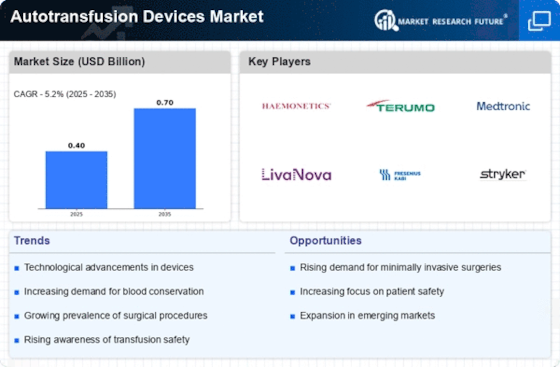
Technological innovations in the field of autotransfusion devices are propelling the growth of the Autotransfusion Devices Market. Recent advancements have led to the development of more efficient and user-friendly devices, enhancing their functionality and reliability. Innovations such as automated blood collection systems and improved filtration techniques have made autotransfusion devices more effective in various surgical settings. Market data suggests that the introduction of these advanced technologies is likely to increase adoption rates among healthcare providers. Furthermore, the integration of digital technologies, such as real-time monitoring and data analytics, is expected to enhance the overall performance of autotransfusion devices. As healthcare facilities seek to improve patient outcomes and streamline operations, the demand for technologically advanced autotransfusion solutions is anticipated to rise.
Increasing Surgical Procedures
The rise in surgical procedures across various medical specialties is a primary driver for the Autotransfusion Devices Market. As surgical interventions become more common, the need for effective blood management solutions intensifies. In recent years, the number of surgeries performed has shown a steady increase, with estimates suggesting that millions of procedures are conducted annually. This trend is likely to continue, as advancements in surgical techniques and technologies facilitate more complex operations. Consequently, the demand for autotransfusion devices, which allow for the collection and reinfusion of a patient's own blood, is expected to grow. This growth is further supported by the increasing awareness of the benefits of autotransfusion, including reduced risk of transfusion-related complications and improved patient outcomes.
Rising Incidence of Trauma Cases
The escalating incidence of trauma cases, including accidents and injuries, significantly influences the Autotransfusion Devices Market. Trauma cases often require immediate surgical intervention, leading to substantial blood loss. Autotransfusion devices play a crucial role in managing this blood loss by enabling the rapid collection and reinfusion of a patient's own blood. Data indicates that trauma-related injuries account for a considerable percentage of emergency room visits and surgical procedures. As the global population continues to grow and urbanization increases, the frequency of trauma cases is likely to rise, thereby driving the demand for autotransfusion devices. This trend underscores the importance of effective blood management solutions in trauma care, positioning autotransfusion devices as essential tools in modern medical practice.
Regulatory Support and Guidelines
Regulatory support and guidelines play a pivotal role in shaping the Autotransfusion Devices Market. Various health authorities and organizations are establishing standards and protocols that encourage the use of autotransfusion devices in clinical practice. These regulations aim to ensure patient safety and promote best practices in blood management. As healthcare facilities strive to comply with these guidelines, the demand for autotransfusion devices is expected to rise. Additionally, regulatory bodies are increasingly recognizing the importance of autotransfusion in reducing reliance on donor blood, which can be scarce in certain regions. This recognition is likely to foster a more favorable environment for the development and adoption of autotransfusion technologies, ultimately contributing to market growth.
Growing Awareness of Blood Conservation
The growing awareness of blood conservation strategies among healthcare professionals and patients is a significant driver for the Autotransfusion Devices Market. As the risks associated with allogeneic blood transfusions become more widely recognized, there is an increasing emphasis on utilizing a patient's own blood whenever possible. This shift in perspective is fostering a greater acceptance of autotransfusion devices as a viable alternative to traditional blood transfusions. Educational initiatives and guidelines from medical organizations are further promoting the benefits of autotransfusion, including reduced transfusion-related complications and improved recovery times. Market analysis indicates that this heightened awareness is likely to lead to increased adoption of autotransfusion devices in surgical and trauma settings, thereby expanding the market.