Rising Prevalence of Autoimmune Diseases
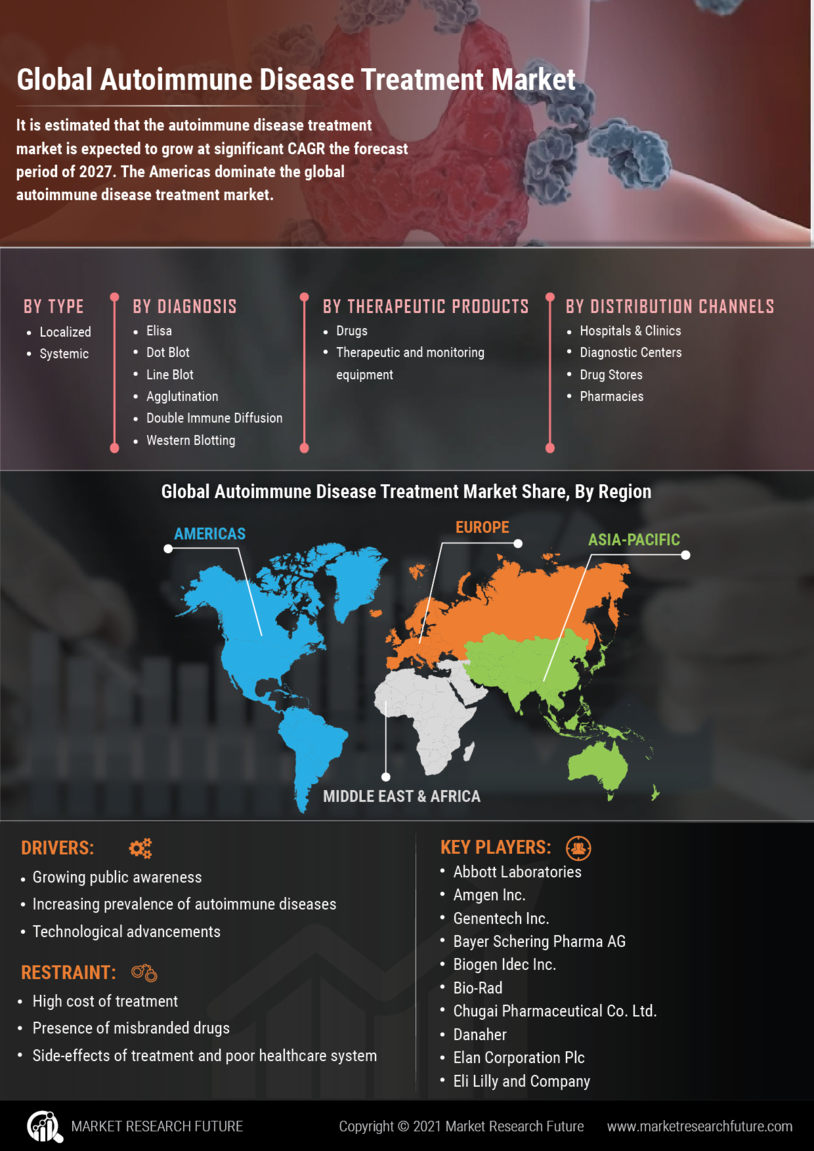
The increasing incidence of autoimmune diseases is a primary driver for the Autoimmune Disease Treatment Market. Conditions such as rheumatoid arthritis, lupus, and multiple sclerosis are becoming more prevalent, affecting millions worldwide. According to recent estimates, autoimmune diseases affect approximately 5-8% of the population, leading to a growing demand for effective treatment options. This rising prevalence necessitates advancements in therapeutic strategies, thereby propelling the market forward. As healthcare systems strive to address this surge, pharmaceutical companies are investing heavily in research and development to create innovative therapies. The focus on personalized medicine further enhances treatment efficacy, catering to the unique needs of patients. Consequently, the Autoimmune Disease Treatment Market is poised for substantial growth as stakeholders respond to the increasing burden of these chronic conditions.
Advancements in Biologics and Targeted Therapies
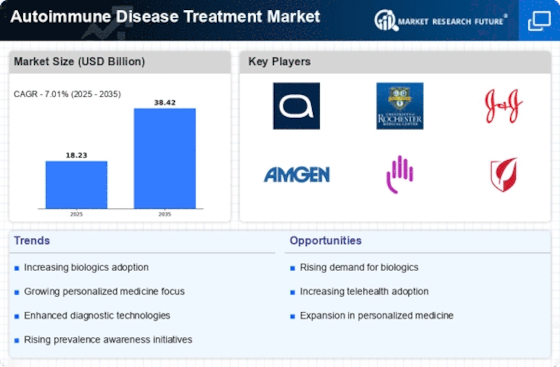
The evolution of biologics and targeted therapies represents a significant driver in the Autoimmune Disease Treatment Market. These innovative treatment modalities have transformed the management of autoimmune diseases, offering more effective and tailored options for patients. Biologics, which are derived from living organisms, have shown remarkable efficacy in treating conditions like rheumatoid arthritis and psoriasis. The market for biologics is projected to reach USD 100 billion by 2025, reflecting the growing acceptance and demand for these therapies. Furthermore, targeted therapies that focus on specific pathways involved in autoimmune responses are gaining traction, providing new avenues for treatment. As research continues to unveil the complexities of autoimmune diseases, the development of these advanced therapies is likely to enhance patient outcomes and drive market growth. The Autoimmune Disease Treatment Market is thus experiencing a paradigm shift towards more sophisticated treatment options.
Increased Investment in Research and Development
Investment in research and development (R&D) is a crucial driver for the Autoimmune Disease Treatment Market. Pharmaceutical companies and research institutions are allocating substantial resources to uncover novel therapeutic targets and improve existing treatments. The global R&D expenditure in the pharmaceutical sector is expected to exceed USD 200 billion by 2025, with a significant portion directed towards autoimmune diseases. This influx of funding facilitates clinical trials, which are essential for validating new therapies and ensuring their safety and efficacy. Moreover, collaborations between academia and industry are fostering innovation, leading to the discovery of breakthrough treatments. As the understanding of autoimmune diseases deepens, the Autoimmune Disease Treatment Market is likely to benefit from a continuous pipeline of new therapies, ultimately enhancing patient care and treatment options.
Aging Population and Increased Healthcare Expenditure
The aging population is a significant driver of the Autoimmune Disease Treatment Market. As individuals age, the risk of developing autoimmune diseases increases, leading to a higher prevalence of these conditions among older adults. This demographic shift is accompanied by increased healthcare expenditure, as older patients often require more comprehensive and long-term treatment plans. According to projections, the global population aged 65 and older is expected to reach 1.5 billion by 2050, further intensifying the demand for effective autoimmune disease treatments. Healthcare systems are adapting to this trend by allocating more resources towards managing chronic conditions, including autoimmune diseases. As a result, the Autoimmune Disease Treatment Market is likely to witness sustained growth, driven by the need for innovative therapies that cater to the unique challenges posed by an aging population.
Growing Awareness and Diagnosis of Autoimmune Diseases
The increasing awareness and improved diagnostic capabilities for autoimmune diseases are driving the Autoimmune Disease Treatment Market. As healthcare professionals and patients become more informed about these conditions, there is a corresponding rise in diagnosis rates. Enhanced diagnostic tools, including advanced imaging techniques and biomarker identification, enable earlier and more accurate detection of autoimmune diseases. This trend is crucial, as early intervention can significantly improve patient outcomes and reduce long-term healthcare costs. The rise in awareness campaigns and educational initiatives is further contributing to this phenomenon, leading to a more proactive approach to managing autoimmune diseases. Consequently, the Autoimmune Disease Treatment Market is experiencing heightened demand for effective treatments, as more individuals seek medical attention for their symptoms.