Regulatory Support and Approvals
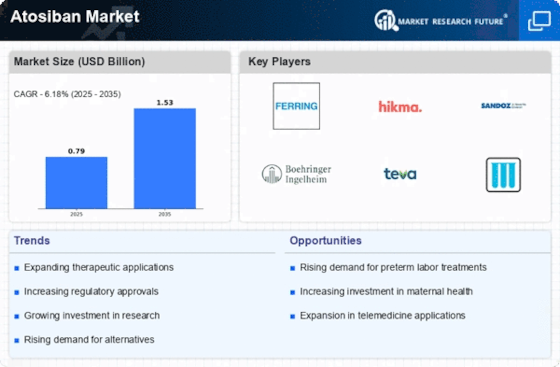
Regulatory support plays a pivotal role in shaping the Atosiban Market. The approval of Atosiban Market by various health authorities has facilitated its entry into multiple markets, thereby enhancing its availability to healthcare providers. Regulatory bodies are increasingly recognizing the importance of tocolytic agents in managing preterm labor, which may lead to streamlined approval processes for new formulations or indications. This supportive regulatory environment is likely to encourage pharmaceutical companies to invest in the development of Atosiban Market, further expanding its market reach. As more countries adopt favorable regulations, the Atosiban Market is expected to experience accelerated growth.
Growing Investment in Maternal Health
Investment in maternal health initiatives is a significant driver for the Atosiban Market. Governments and non-governmental organizations are increasingly allocating resources to improve maternal care, particularly in regions with high rates of preterm births. This focus on maternal health is likely to lead to greater accessibility and availability of Atosiban Market, as healthcare systems prioritize effective interventions for preterm labor. The integration of Atosiban Market into maternal health programs may also be supported by training healthcare providers on its use, thereby enhancing its market presence. As funding for maternal health continues to grow, the Atosiban Market stands to benefit from increased utilization and awareness.
Advancements in Pharmaceutical Research
Innovations in pharmaceutical research are propelling the Atosiban Market forward. Ongoing studies are exploring the efficacy and safety of Atosiban Market in various clinical settings, which may lead to expanded indications for its use. The development of new formulations and delivery methods could enhance patient compliance and therapeutic outcomes. Furthermore, research into the pharmacogenomics of Atosiban Market may provide insights into personalized medicine approaches, potentially increasing its market penetration. As the scientific community continues to validate the benefits of Atosiban Market, the market is likely to experience a positive trajectory, supported by evidence-based practices and enhanced treatment protocols.
Increasing Prevalence of Preterm Births
The rising incidence of preterm births is a critical driver for the Atosiban Market. According to recent data, approximately 15 million infants are born preterm each year, which represents a significant public health challenge. This trend is likely to escalate the demand for effective tocolytic agents like Atosiban Market, which is utilized to manage preterm labor. As healthcare providers seek to mitigate the risks associated with preterm deliveries, the Atosiban Market is poised for growth. The increasing awareness among healthcare professionals regarding the benefits of Atosiban Market in prolonging pregnancy duration may further enhance its adoption. Consequently, the market is expected to witness a surge in demand, driven by both clinical guidelines and patient needs.
Rising Awareness Among Healthcare Professionals
The increasing awareness among healthcare professionals regarding the benefits of Atosiban Market is a crucial driver for the Atosiban Market. Educational initiatives and clinical guidelines are emphasizing the importance of effective management of preterm labor, which is likely to enhance the adoption of Atosiban Market in clinical practice. As obstetricians and gynecologists become more informed about the advantages of using Atosiban Market over traditional tocolytics, its utilization may rise significantly. This heightened awareness could lead to a shift in prescribing patterns, favoring Atosiban Market as a first-line treatment option. Consequently, the Atosiban Market may witness a robust growth trajectory, driven by informed clinical decision-making.