Rising Fertility Awareness in APAC
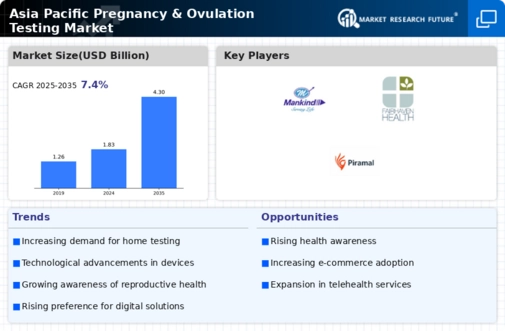
The APAC Pregnancy Ovulation Testing Market is experiencing a notable increase in fertility awareness among women. This trend is largely driven by enhanced access to information through digital platforms and social media. Women are becoming more informed about their reproductive health, leading to a greater demand for ovulation testing products. According to recent data, the market for ovulation tests in the APAC region is projected to grow at a compound annual growth rate (CAGR) of approximately 8% over the next five years. This growth is indicative of a shift towards proactive family planning, as women seek to optimize their chances of conception. As awareness continues to rise, the APAC Pregnancy Ovulation Testing Market is likely to see an influx of innovative products tailored to meet the needs of this increasingly informed demographic.
Cultural Acceptance of Fertility Testing
Cultural attitudes towards fertility testing are evolving within the APAC region, positively impacting the Pregnancy Ovulation Testing Market. Traditionally, discussions around reproductive health were often stigmatized, but there is a growing acceptance of fertility-related topics. This cultural shift is encouraging women to seek out ovulation testing as a means of understanding their reproductive health. Countries such as South Korea and Singapore are witnessing a rise in the normalization of fertility discussions, leading to increased demand for ovulation testing products. As societal norms continue to change, the APAC Pregnancy Ovulation Testing Market is likely to benefit from a broader acceptance of fertility testing, which could further drive market growth.
Technological Innovations in Testing Devices
Technological advancements are significantly influencing the APAC Pregnancy Ovulation Testing Market. The introduction of digital and smartphone-compatible ovulation testing devices is revolutionizing how women track their fertility. These innovations offer greater accuracy and convenience, appealing to tech-savvy consumers. For example, some devices now utilize advanced algorithms to predict ovulation more precisely, which can enhance the chances of conception. The market is witnessing a surge in demand for these high-tech solutions, with projections indicating that the segment for digital ovulation tests could account for over 30% of the total market share by 2028. As technology continues to evolve, the APAC Pregnancy Ovulation Testing Market is likely to see further innovations that cater to the needs of modern women.
Government Initiatives Supporting Family Planning
Government initiatives across various APAC countries are playing a crucial role in shaping the Pregnancy Ovulation Testing Market. Many governments are implementing policies aimed at promoting family planning and reproductive health. For instance, countries like India and Indonesia have launched campaigns to educate women about fertility and the importance of ovulation tracking. These initiatives not only raise awareness but also encourage the use of ovulation testing kits. The APAC Pregnancy Ovulation Testing Market is expected to benefit from these supportive policies, as they create a conducive environment for the growth of fertility-related products. Furthermore, the collaboration between public health organizations and private sector companies is likely to enhance the distribution and accessibility of ovulation testing kits, thereby expanding the market reach.
Growing E-commerce Platforms for Product Accessibility
The rise of e-commerce platforms is transforming the APAC Pregnancy Ovulation Testing Market by enhancing product accessibility. With the increasing penetration of the internet and mobile devices, consumers are turning to online shopping for health-related products, including ovulation testing kits. This shift is particularly evident in countries like China and Japan, where online sales of health products have surged. E-commerce not only provides convenience but also allows for a wider range of products to be available to consumers. Market data suggests that online sales could represent nearly 40% of the total sales in the APAC Pregnancy Ovulation Testing Market by 2026. This trend indicates a significant shift in consumer behavior, as women seek easy access to reliable ovulation testing solutions.