Market Analysis
In-depth Analysis of Aseptic Sampling Market Industry Landscape
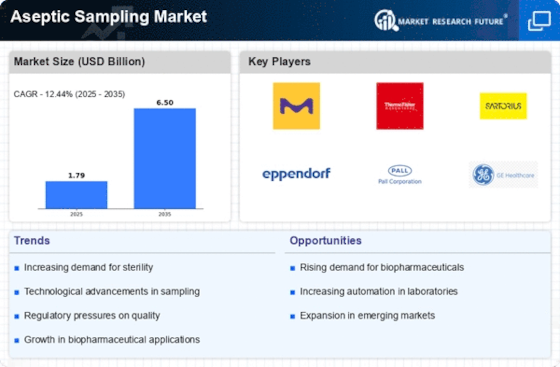
Because it maintains sterile conditions during the sample process, the aseptic sampling market is essential to the biotechnology and pharmaceutical sectors. A number of variables, including regulatory constraints and technical improvements, impact market dynamics in this industry. Growing Requirement for Aseptic Methods One of the main factors propelling the market's expansion is the growing need for aseptic methods in the bioprocessing and pharmaceutical industries. In order to avoid contamination, guarantee product quality, and adhere to strict regulatory requirements, aseptic sampling is essential. Technological Developments: The field of aseptic sampling is changing as a result of ongoing technological developments. With their increased efficiency and lower danger of contamination, innovations like automated aseptic sampling devices, closed-loop sampling, and single-use systems are becoming more and more popular. Strict Regulations: The aseptic sampling industry is growing as a result of the strict regulations that health authorities throughout the world have established. Adherence to regulatory criteria issued by regulatory agencies, including the FDA and the European Medicines Agency (EMA), is crucial for businesses functioning in this domain and propels the uptake of sophisticated aseptic sample systems. Growth of the Biopharmaceutical Industry: One major driver affecting the aseptic sample market is the biopharmaceutical industry's expansion, which is centered on biologics and customized medicine. As bioprocessing becomes increasingly common, there is an increasing demand for aseptic sampling methods that are dependable and effective. Raising the Bar for Quality Assurance In the production of pharmaceuticals and biotechnology, quality control is crucial. Because aseptic sampling is essential to guaranteeing the quality and safety of products, there is a greater focus on implementing. Globalization of Pharmaceutical Manufacturing: Production facilities have become more decentralized as a result of the globalization of pharmaceutical manufacturing. As a result, there is now a larger need for standardized aseptic sampling techniques across a wider range of geographic areas, which is driving market expansion. Cost-Effective Single-Use Systems: Aseptic sampling is increasingly adopting cost-effective single-use systems. The market acceptance of these systems is fueled by the fact that they do not require cleaning or sterilization, which lowers the danger of cross-contamination and provides cost savings. Difficulties with Implementation: Although the market dynamics are favorable, there are still difficulties with implementing aseptic sampling methods. These include of the upfront financial outlay, the requirement for specific training, and possible opposition to modifications in conventional production procedures.

















Leave a Comment