Market Trends
Key Emerging Trends in the Artificial Coma Medically Induced Coma Market
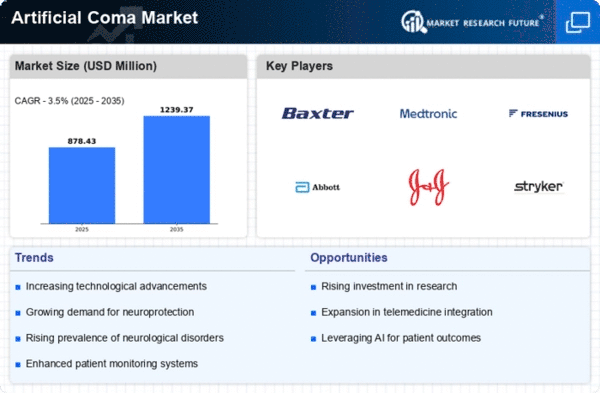
Artificial Coma/Medically Induced Coma Market has shown remarkable signs of development that mirror the progress in medical care, brain function advances in neurological interventions. It is a procedure where medicines are given to make patients unconscious, usually those who are critically ill, with intentions of protecting the brain and facilitating medical procedures. Artificial Coma/Medically Induced Coma Market trends over recent years have been defined by some key factors including changes in clinical practices, technology, and better patient outcomes.
One prominent trend shaping the Artificial Coma/Medically Induced Coma Market is increased use of induced coma as a therapeutic strategy in critical care settings. The medically induced coma traditionally used for traumatic brain injuries and certain neurological conditions is being tested for its use in other areas of critical care such as post-cardiac arrest care and refractory status epilepticus. This trend shows the shift from generic approaches to more individualized induction of comas based on specific needs of affected patients.
Technological advancements in monitoring and life support systems drive innovation within Artificial Coma/Medically Induced Coma Market. Integration of advanced monitoring devices enables healthcare providers to closely monitor physiological parameters, brain function, and response to induced coma. These technological advances result in a more personalized approach to managing induced coma thus allowing real-time adjustments that optimize therapy while reducing risks.
Moreover, there is an increasing focus on research studies and clinical trials aimed at understanding more about the mechanisms behind induced coma as well as its potential benefits. As knowledge on neuroprotectiveity effects of induced coma diversifies, interest grows for finding new indications or adapting administration protocols leading to better management of this condition . Collaborations between academic institutions, healthcare facilities together with pharmaceutical companies have led them into conducting researches regarding various aspects associated with the use of these comas as therapeutic instruments.
The current trend in the artificial/medicinally-induced markets toward safer sedative agents with improved pharmacokinetic profiles and fewer side effects. In order to obtain safer and controllable sedation during induced coma, new drugs have been investigated that can strike a balance between getting the desired therapeutic effect and minimizing possible complications. A broader choice of sedatives allows doctors to individualize regimens of induced coma thus contributing to its general safety and effectiveness.
Additionally, there is an increased awareness and emphasis on ethical considerations as well as patient/family engagement in the decision-making process regarding induced coma. Since induced coma involves complex medical decisions that touch on ethics, health providers are currently giving more weight to transparency in communication, shared decision making, as well as providing patients and families with full information about risks and benefits associated with this procedure. This has led to a trend towards patient-centered care in healthcare generally where ethical practices are also being encouraged within critical care interventions.”
Artificial Coma/Medically Induced Coma Market Despite these positive trends, there are still some challenges in the artificial coma/medically induced coma market. The long-term neurological outcomes of these comas, the variations in clinical practices and ethical issues concerning their use in certain situations are among the persisting concerns. In addressing these challenges and advancing comprehension and application of induced coma in critical care, this requires ongoing research, standardization of protocols as well as interdisciplinary collaboration.

















Leave a Comment