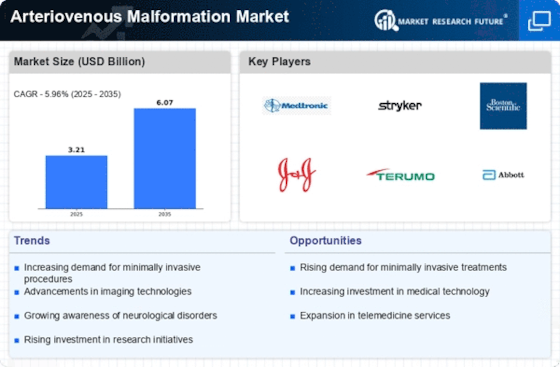
Top Industry Leaders in the Arteriovenous Malformation Market
 Latest Arteriovenous malformation Companies Update
Latest Arteriovenous malformation Companies Update
January 2023: Eisai Co., Ltd. and Biogen Inc. jointly declared that the U.S. Food and Drug Administration (FDA) had granted approval for lecanemab-irmb 100 mg/mL injection for intravenous administration via the Accelerated Approval Pathway. Lecanemab-irmb is a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody designed to target aggregated soluble and insoluble forms of amyloid beta (Aβ). It is indicated for the treatment of Alzheimer's disease (AD). The approval is predicated on Phase 2 data indicating that LEQEMBI decreased the deposition of Aβ plaque in the brain, which is a characteristic feature of Alzheimer's disease. Eisai will expeditiously employ the data recently disclosed from the expansive worldwide confirmatory Phase 3 clinical trial, Clarity AD, to submit a Supplemental Biologics License Application (sBLA) to the FDA in pursuit of conventional pathway approval.
August 2022: Vaderis Therapeutics AG emerged from covert mode with a clinical-ready drug candidate and an emphasis on allosteric AKT inhibitors, thanks to financial backing from Medicxi. The nascent biotechnology company intends to initiate a proof-of-concept clinical trial to evaluate its principal compound VAD044 in individuals afflicted with Hereditary Hemorrhagic Telangiectasia (HHT). HHT, which is also referred to as Osler-Weber-Rendu Syndrome, is a hereditary condition characterized by the formation of arteriovenous malformations, which are aberrant connections between veins and arteries. Nose, lung, brain, and liver are the organs most frequently impacted by these malformations. The malformations may eventually induce spontaneous hemorrhage. Anemia, iron deficiency, shortness of breath, and seizures are all symptoms of the disease that can negatively impact quality of life. At this time, no pharmaceuticals have been approved by the FDA for the specific purpose of treating HHT. Vaderis aspires to be the first organization to transport a candidate for this disease through the clinic.
List of Arteriovenous Malformation Key companies in the market
- Toshiba Medical Systems Corporation (Japan)
- Fujifilm Holdings (Japan)
- Carestream Health (U.S.)
- Electrical Geodesics Inc. (U.S.)
- Nihon Kohden Corporation (Japan)