Technological Advancements
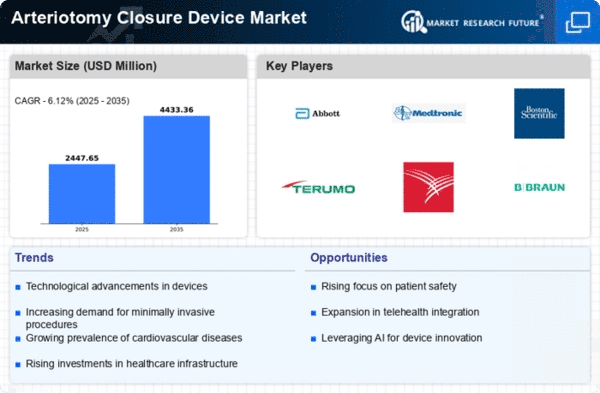
Advancements in medical technology significantly enhance the efficacy and safety of arteriotomy closure devices. Innovations such as bioabsorbable materials and advanced suturing techniques improve patient outcomes and reduce complications. The Global Arteriotomy Closure Device Market Industry benefits from these developments, as healthcare providers increasingly adopt cutting-edge solutions to optimize surgical procedures. For instance, devices that offer faster closure times and reduced risk of infection are gaining traction. This trend is likely to propel the market forward, with projections indicating a growth trajectory that could reach 4.44 USD Billion by 2035, reflecting a compound annual growth rate of 6.11% from 2025 to 2035.
Growing Geriatric Population
The global increase in the geriatric population is a crucial driver for the arteriotomy closure device market. Older adults are more susceptible to cardiovascular diseases, necessitating surgical interventions that often require effective closure solutions. The Global Arteriotomy Closure Device Market Industry is poised to expand as healthcare systems adapt to the needs of this demographic. With the aging population projected to rise significantly in the coming years, the demand for arteriotomy closure devices is likely to increase correspondingly. This demographic shift underscores the importance of developing specialized devices that cater to the unique requirements of elderly patients undergoing cardiovascular procedures.
Rising Healthcare Expenditure
Increased healthcare expenditure across various regions contributes to the growth of the arteriotomy closure device market. Governments and private sectors are investing more in healthcare infrastructure, leading to enhanced surgical capabilities and access to advanced medical devices. The Global Arteriotomy Closure Device Market Industry stands to benefit from this trend, as hospitals and clinics seek to upgrade their surgical equipment. This investment in healthcare is expected to facilitate the adoption of innovative closure devices, ultimately improving patient care. As healthcare budgets expand, the market is likely to experience sustained growth, aligning with the increasing demand for effective surgical solutions.
Regulatory Support and Guidelines
Supportive regulatory frameworks and guidelines play a pivotal role in shaping the arteriotomy closure device market. Regulatory bodies are increasingly recognizing the importance of these devices in improving surgical outcomes and patient safety. The Global Arteriotomy Closure Device Market Industry is positively influenced by streamlined approval processes and updated guidelines that encourage the adoption of new technologies. This regulatory support fosters innovation and instills confidence among healthcare providers, leading to greater utilization of arteriotomy closure devices in clinical practice. As regulations evolve to support advanced medical solutions, the market is expected to thrive in response to these favorable conditions.
Increasing Cardiovascular Diseases
The rising prevalence of cardiovascular diseases globally drives the demand for arteriotomy closure devices. As per health statistics, cardiovascular diseases remain a leading cause of mortality worldwide. This trend necessitates effective surgical interventions, thereby increasing the utilization of arteriotomy closure devices in procedures such as angioplasty and stent placements. The Global Arteriotomy Closure Device Market Industry is expected to witness a surge in demand as healthcare providers prioritize patient safety and recovery. This growing focus on minimally invasive techniques further emphasizes the need for reliable closure devices, contributing to the market's projected growth to 2.31 USD Billion in 2024.