-
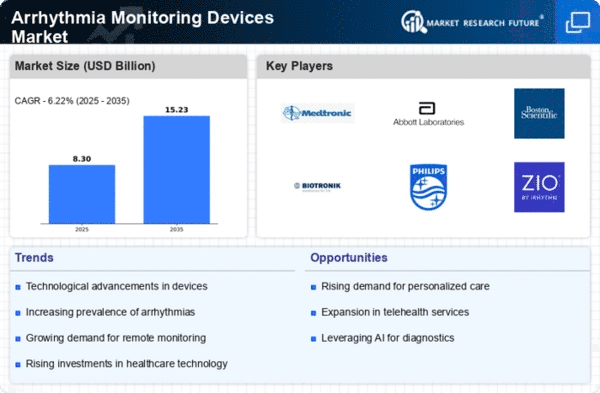
Executive Summary
-
Market Introduction
-
Definition
-
Scope of the Study
-
Market Structure
-
Research Methodology
-
Research Process
-
Primary Research
-
Secondary Research
-
Market Size Estimation
-
Forecast Model
-
Market Dynamics
-
Overview
-
Drivers
- Rising Prevalence Rate of Cardiac Diseases
- Increasing FDA Approvals
- Surge in the Adoption of Low-Cost Electrocardiogram (ECG) Devices
- Increasing Geriatric Population
-
Restraints
- Increase in Product Recalls
- Complications Related to Devices
-
Opportunities
- Increasing Technological Advancements
-
Market Factor Analysis
-
Value Chain Analysis
- R&D and Designing
- Manufacturing
- Distribution & Sales
- Post-Sales Review
-
Porter’s Five Forces Model
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Intense Rivalry
-
Arrhythmia Monitoring Devices Market, by Device Type
-
Overview
-
Resting ECG
-
Event Monitor
-
Implantable Cardiac Monitors (ICM)
-
Holter Monitor
-
Mobile Cardiac Telemetry (MCT) Devices
-
Others
-
Arrhythmia Monitoring Devices Market, by Application
-
Overview
-
Tachycardia
-
Bradycardia
-
Atrial Fibrillation (A-fib)
-
Ventricular Fibrillation (V-fib)
-
Premature Ventricular Contractions (PVC)
-
Conduction Disorders
-
Others
-
Arrhythmia Monitoring Devices Market, by End User
-
Overview
-
Hospitals & Clinics
-
Diagnostic Centers
-
Homecare Settings
-
Ambulatory Surgery Centers (ASC)
-
Others
-
Global Arrhythmia Monitoring Devices Market, by Region
-
Overview
-
Americas
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
North America
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
US
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Canada
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Latin America
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Brazil
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Rest of Latin America
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Europe
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Western Europe
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Germany
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
UK
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
France
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Italy
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Spain
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Rest of Western Europe
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Eastern Europe
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Asia-Pacific
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Japan
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
China
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
India
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Australia
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
South Korea
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Rest of Asia-Pacific
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Middle East & Africa
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Middle East
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Africa
-
Arrhythmia Monitoring Devices Market, by Device Type Arrhythmia Monitoring Devices Market, by Application Arrhythmia Monitoring Devices Market, by End User
-
Competitive Landscape
-
Overview
-
Company Share Analysis
-
Company Profiles
-
Biotronik
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Abbott Laboratories
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Medtronic plc
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
GE Healthcare
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
BioTelemetry, Inc.
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Applied Cardiac Systems, Inc.
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
AliveCor, Inc.
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Nihon Kohden Corporation
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Fukuda Denshi Co. Ltd
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Spacelabs Healthcare
- Company Overview
- Financial Overview
- Products/Services Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Appendix
-
References
-
Related Reports
-
List of Tables
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR RESTING ECG, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR EVENT MONITORS, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR IMPLANTABLE CARDIAC MONITORS (ICM), BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR HOLTER MONITORS, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR MOBILE CARDIAC TELEMETRY (MCT) DEVICES, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR TACHYCARDIA, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR BRADYCARDIA, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR ATRIAL FIBRILLATION (A-FIB), BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR VENTRICULAR FIBRILLATION (V-FIB), BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR PREMATURE VENTRICULAR CONTRACTIONS (PVC), BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR CONDUCTION DISORDERS, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR HOSPITALS AND CLINICS, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR DIAGNOSTIC CENTERS, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR HOMECARE SETTINGS, BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET FOR AMBULATORY SURGERY CENTERS (ASC), BY REGION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION, 2020–2027 (USD MILLION)
-
AMERICAS: ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION, 2020–2027 (USD MILLION)
-
AMERICAS: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
AMERICAS: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
AMERICAS: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
NORTH AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
-
NORTH AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
NORTH AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
NORTH AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
US: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
US: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
US: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
CANADA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
CANADA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
CANADA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
-
LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
BRAZIL: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
BRAZIL: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
BRAZIL: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
REST OF LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
REST OF LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
REST OF LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION, 2020–2027 (USD MILLION)
-
EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
-
WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
GERMANY: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
GERMANY: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
GERMANY: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
UK: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
UK: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
UK: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
FRANCE: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
FRANCE: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
FRANCE: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
ITALY: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
ITALY: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
ITALY: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
SPAIN: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
SPAIN: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
SPAIN: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
REST OF WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
REST OF WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
REST OF WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
EASTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
EASTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
EASTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
-
ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
JAPAN: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
JAPAN: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
JAPAN: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
CHINA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
CHINA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
CHINA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
INDIA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
INDIA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
INDIA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
AUSTRALIA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
AUSTRALIA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
AUSTRALIA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
SOUTH KOREA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
SOUTH KOREA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
SOUTH KOREA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
REST OF ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
REST OF ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
REST OF ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
MIDDLE EAST & AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION, 2020–2027 (USD MILLION)
-
MIDDLE EAST & AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
MIDDLE EAST & AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
MIDDLE EAST & AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
MIDDLE EAST: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
MIDDLE EAST: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
MIDDLE EAST: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
List of Figures
-
MARKET SYNOPSIS
-
MARKET ATTRACTIVENESS ANALYSIS: GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE, BY DEVICE TYPE,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE, BY APPLICATION,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE, BY END USER,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER, 2020–2027 (USD MILLION)
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET: MARKET STRUCTURE
-
RESEARCH PROCESS OF MRFR
-
TOP-DOWN & BOTTOM-UP APPROACH
-
MARKET DYNAMICS: ANALYSIS OF THE GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET
-
DRIVERS IMPACT ANALYSIS: GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET
-
RESTRAINTS IMPACT ANALYSIS: GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET
-
VALUE CHAIN: GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET
-
PORTER’S FIVE FORCES ANALYSIS: GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE, BY DEVICE TYPE,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY DEVICE TYPE,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE, BY APPLICATION,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY APPLICATION,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE, BY END USER,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY END USER,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE, BY REGION,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION,
-
AMERICAS: ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION,
-
NORTH AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY,
-
LATIN AMERICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY,
-
EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION,
-
WESTERN EUROPE: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY,
-
ASIA-PACIFIC: ARRHYTHMIA MONITORING DEVICES MARKET, BY COUNTRY,
-
MIDDLE EAST & AFRICA: ARRHYTHMIA MONITORING DEVICES MARKET, BY REGION,
-
GLOBAL ARRHYTHMIA MONITORING DEVICES MARKET SHARE ANALYSIS,





Leave a Comment