Growing Regulatory Pressure
Growing regulatory pressure is a significant factor impacting the Analytical Chromatography In Pharma Quality Control Market. Regulatory agencies are increasingly demanding rigorous testing and validation processes to ensure drug safety and efficacy. This has led to a surge in the adoption of analytical chromatography techniques, which are essential for meeting these regulatory requirements. The market is likely to see continued growth as pharmaceutical companies strive to comply with evolving regulations. The need for reliable and efficient quality control measures is paramount, indicating a robust future for analytical chromatography solutions in the pharmaceutical sector.
Expansion of Biopharmaceuticals
The expansion of biopharmaceuticals is reshaping the Analytical Chromatography In Pharma Quality Control Market. As the biopharmaceutical sector continues to grow, driven by innovations in drug development, the demand for sophisticated analytical techniques becomes paramount. Analytical chromatography plays a crucial role in the characterization and quality assessment of biopharmaceutical products. The market for biopharmaceuticals is expected to reach over 400 billion USD by 2025, which in turn fuels the need for robust quality control measures. This trend indicates a sustained demand for analytical chromatography solutions that can meet the unique challenges posed by biologics.
Rising Demand for Quality Assurance
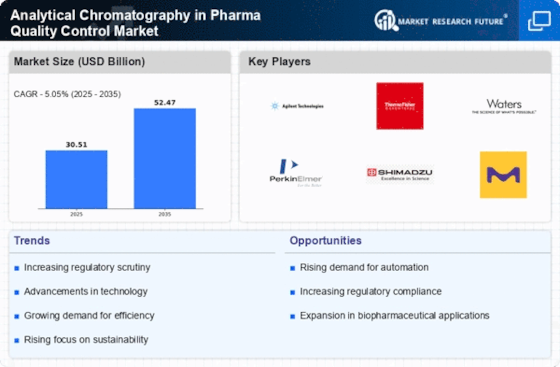
The increasing emphasis on quality assurance in the pharmaceutical sector is a primary driver for the Analytical Chromatography in Pharma Quality Control Market. As regulatory bodies enforce stricter guidelines, pharmaceutical companies are compelled to adopt advanced analytical techniques to ensure product safety and efficacy. The market for analytical chromatography is projected to grow significantly, with estimates suggesting a compound annual growth rate of over 6% in the coming years. This growth is largely attributed to the need for precise and reliable testing methods that analytical chromatography provides, thereby enhancing the overall quality control processes in pharmaceutical manufacturing.
Emergence of Advanced Analytical Techniques
The emergence of advanced analytical techniques is a notable driver for the Analytical Chromatography In Pharma Quality Control Market. Innovations such as ultra-high-performance liquid chromatography (UHPLC) and two-dimensional chromatography are enhancing the capabilities of traditional methods. These advancements allow for faster analysis times and improved resolution, which are critical in the fast-paced pharmaceutical environment. The market for these advanced techniques is expected to grow, as they provide pharmaceutical companies with the tools necessary to meet stringent quality control requirements. This trend suggests a shift towards more sophisticated analytical methodologies in the industry.
Increased Focus on Research and Development
The heightened focus on research and development within the pharmaceutical industry significantly influences the Analytical Chromatography In Pharma Quality Control Market. As companies invest more in R&D to innovate and develop new drugs, the necessity for reliable analytical methods becomes increasingly critical. Analytical chromatography is essential for the analysis of complex mixtures and the identification of active pharmaceutical ingredients. With R&D spending projected to exceed 200 billion USD annually, the demand for analytical chromatography solutions is likely to rise, as these techniques are integral to ensuring compliance with quality standards throughout the drug development process.