Market Share
Actinic Keratosis Treatment Market Share Analysis
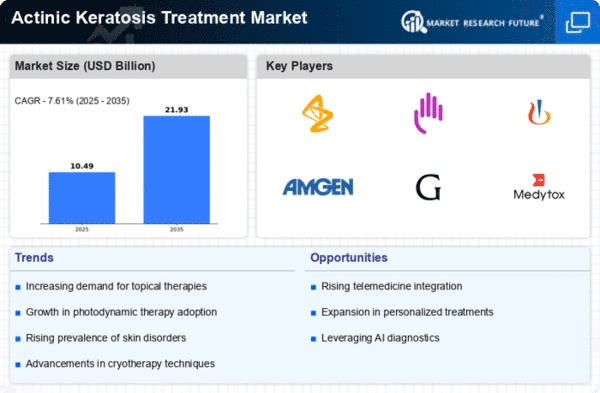
We took a closer look at the big companies that play a key role in treating actinic keratosis. Some of the major players include Biofrontera AG, Cipher Pharmaceuticals Inc., BioLineRx, Valeant Pharmaceuticals, Leo Pharma, Galderma S.A, Sun Pharmaceutical Industries Ltd, 3M, Alma Lasers, Stanford Chemicals, and a few others. Alongside these, there are many smaller companies operating in this field. These smaller companies usually focus on specific regions and serve a particular group of customers. We've examined the financial growth of both big and small companies and evaluated how they compete in the market. By considering the global and regional sales of their products, we've estimated the market share held by these companies on a worldwide scale.
It's important to note that the actinic keratosis treatment market involves not only the big players but also numerous smaller and medium-sized companies. These smaller companies, while not as widely known, play a significant role by catering to specific regions and meeting the needs of a particular customer base. In addition to the major players like Biofrontera AG, Cipher Pharmaceuticals Inc., and others, there are various small-scale enterprises that contribute to the market's diversity.
We dug deep into the financial performance of both large and small companies in this market. This analysis helps us understand how well they are doing in terms of growth and sustainability. The market competition, a crucial aspect, was thoroughly assessed to provide a comprehensive picture. To determine the market share of each company on a global scale, we considered their product sales not only globally but also regionally.
In essence, the actinic keratosis treatment market is shaped not just by a few major names but by a combination of big and small players. This diversity ensures that different regions and specific customer needs are addressed effectively. By delving into the financial aspects and market competition, we gain valuable insights into the dynamics of this market and how each player contributes to its overall landscape.






Leave a Comment