Innovative Drug Development
The Acromegaly Market is witnessing a wave of innovative drug development, which serves as a significant market driver. Pharmaceutical companies are increasingly investing in research and development to create novel therapies that target the underlying causes of acromegaly. For instance, the introduction of somatostatin analogs and growth hormone receptor antagonists has transformed treatment paradigms. These advancements not only improve patient outcomes but also enhance the overall market landscape. The global market for acromegaly treatments is projected to reach several billion dollars by the end of the decade, driven by these innovative therapies. As new drugs receive regulatory approval, they are likely to capture market share, further stimulating growth in the Acromegaly Market.
Rising Healthcare Expenditure
An increase in healthcare expenditure is another crucial driver for the Acromegaly Market. As countries allocate more resources to healthcare, there is a corresponding rise in the availability of advanced treatment options for acromegaly. This trend is particularly evident in developed regions, where healthcare budgets are expanding to accommodate innovative therapies. The World Health Organization indicates that healthcare spending is expected to grow at a rate of 5% annually, which could facilitate better access to acromegaly treatments. Enhanced funding for healthcare initiatives may also lead to improved diagnostic capabilities, allowing for earlier detection and treatment of acromegaly. Consequently, this increased expenditure is likely to bolster the Acromegaly Market.
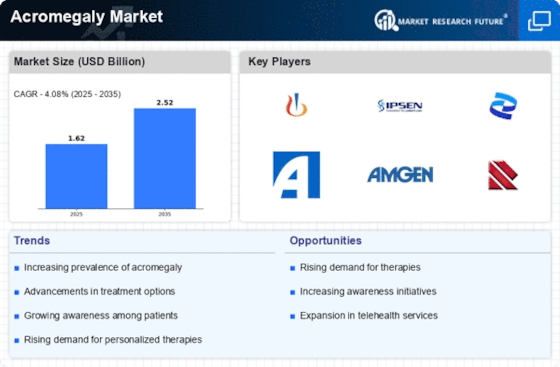
Increasing Prevalence of Acromegaly
The rising incidence of acromegaly is a pivotal driver in the Acromegaly Market. Recent estimates suggest that the prevalence of this disorder is approximately 3 to 4 cases per million people annually. This increase in diagnosed cases is likely to spur demand for treatment options, thereby expanding the market. As awareness grows among healthcare professionals and patients alike, more individuals are being diagnosed, which could lead to a surge in the need for effective therapies. Furthermore, the aging population may contribute to a higher prevalence of acromegaly, as the condition is often linked to pituitary tumors that develop over time. Consequently, the Acromegaly Market is poised for growth as healthcare systems adapt to manage this increasing patient population.
Growing Focus on Patient-Centric Care
The Acromegaly Market is increasingly influenced by a growing focus on patient-centric care. Healthcare providers are recognizing the importance of tailoring treatment plans to individual patient needs, which is reshaping the market landscape. This shift towards personalized medicine is likely to enhance patient adherence to treatment regimens, ultimately improving health outcomes. Moreover, patient advocacy groups are playing a vital role in raising awareness about acromegaly, which may lead to more patients seeking treatment. As healthcare systems prioritize patient engagement and satisfaction, the demand for customized therapies is expected to rise, thereby driving growth in the Acromegaly Market.
Technological Advancements in Diagnostics
Technological advancements in diagnostic tools are significantly impacting the Acromegaly Market. Enhanced imaging techniques and biomarker identification are facilitating earlier and more accurate diagnoses of acromegaly. For instance, the use of MRI and CT scans has become more prevalent, allowing for better visualization of pituitary tumors. This improved diagnostic capability is likely to lead to an increase in the number of diagnosed cases, subsequently driving demand for treatment options. Furthermore, the integration of artificial intelligence in diagnostic processes may streamline patient assessment, making it easier for healthcare providers to identify acromegaly. As diagnostic technologies continue to evolve, they are expected to play a crucial role in shaping the future of the Acromegaly Market.