Summary Overview
In-Vitro Toxicology Market Overview
The global in-vitro toxicology market is expanding steadily, fuelled by demand from a variety of industries such as pharmaceuticals, chemicals, environmental safety, and consumer goods. This market covers a wide range of in-vitro testing options, including cell-based assays, high-throughput screening, and organ-on-a-chip systems. Our research includes a comprehensive overview of industry trends, including cost-cutting tactics and the use of new digital tools to improve toxicological testing and regulatory processes. Future issues in in-vitro toxicology include controlling high testing costs, assuring the scalability of new technologies, maintaining data quality, and incorporating these testing methods into existing regulatory frameworks. Adopting digital toxicology technologies, together with strategic innovation in test techniques, is critical for enhancing testing efficiency, lowering time-to-market, and assuring compliance with stringent global regulations. As global demand for safer and more reliable product testing grows, organizations are increasingly using market information to improve operational efficiency, assure regulatory compliance, and eliminate product safety concerns.
Market Size The global In-Vitro Toxicology market is projected to reach USD 49.06 billion by 2035, growing at a CAGR of approximately 11.22% from 2025 to 2035.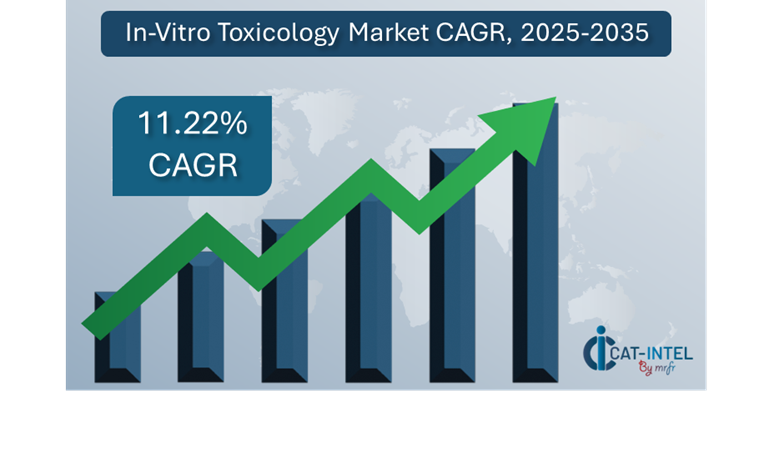
Growth Rate 11.22%
Sector Contributions Growth in the market is driven by
-
Manufacturing and Toxicology Optimization There is a growing need for real-time data and process integration to improve safety and risk assessment in product development and environmental testing. -
Pharmaceutical and Consumer Goods Growth Adoption of in-vitro toxicity testing technologies improves risk assessment, safety practices, and regulatory compliance. -
Technological Advancements Advances in artificial intelligence and machine learning improve in-vitro testing capabilities, allowing for predictive toxicity and automated safety assessments. -
Innovations Modular in-vitro testing solutions enable firms to pick and integrate only the functionalities required, lowering costs and increasing testing efficiency. -
Investment Initiatives Increased investment into digital in-vitro toxicology platforms and modern laboratory technology to shorten testing times, increase accuracy, and maintain regulatory compliance. -
Regional Insights North America and Europe continue to be major market contributors, owing to robust healthcare infrastructure, regulatory frameworks, and increased usage of AI-powered toxicity testing solutions.
Key Trends and Sustainability Outlook
-
Cloud Integration Toxicology solutions are increasingly used for scalable, cost-effective, and real-time data access across research labs and testing facilities. -
Advanced Features The integration of AI, IoT, and blockchain technologies improves toxicological decision-making by increasing automation, transparency, and traceability in testing and reporting. -
Sustainability Focus In-vitro toxicology solutions help to reduce the environmental impact of testing by limiting animal use and improving resource management, hence supporting sustainability goals.
-
Customization Trends There is an increasing need for sector-specific toxicity testing modules suited for pharmaceuticals, cosmetics, and chemicals, which address each industry's particular safety and regulatory requirements. -
Data-Driven Insights Advanced analytics built into in-vitro testing platforms allow organizations to predict toxicity consequences, optimize safety measures, and improve regulatory reporting
Growth Drivers
-
Digital Transformation The increasing use of digital technology in toxicology research and testing to improve productivity, accuracy, and efficiency when determining product safety.
-
Demand for Automation There is an increasing reliance on automated in-vitro testing technologies to streamline repetitious operations, eliminate human error, and shorten testing cycles.
-
Scalability Requirements Companies want scalable in-vitro testing platforms that can expand with their operations, allowing smooth integration into changing regulatory landscapes and research workflows. -
Regulatory Compliance In-vitro toxicity testing platforms help firms comply with regulatory norms by automating compliance processes and ensuring accuracy in reporting.
-
Globalization There is a growing demand for toxicological solutions that can meet multi-region testing requirements, such as compliance with worldwide safety standards, differing regulatory rules, and language
Overview of Market Intelligence Services for the In-Vitro Toxicology Market
Recent evaluations have revealed numerous significant difficulties in the in-vitro toxicology market, including high testing costs and the requirement for system modification. Market intelligence reports provide useful insights into cost-cutting options, assisting organizations in optimizing testing processes, improving supplier management, and ensuring the success of in-vitro toxicology solutions. These insights also assure compliance with industry laws and promote high-quality testing processes, all while keeping expenses under control.
Procurement Intelligence for In-Vitro Toxicology Category Management and Strategic Sourcing
To remain competitive in the in-vitro toxicology market, businesses are improving procurement processes through spend analysis and supplier performance evaluation. Effective category management and smart sourcing are key for reducing testing costs, providing access to high-quality in-vitro testing technologies. Businesses that use actionable market intelligence can improve their procurement strategy, negotiate better rates for in-vitro testing solutions, and obtain dependable suppliers.
Pricing Outlook for In-Vitro Toxicology Spend Analysis
The price prognosis for in-vitro toxicology solutions is projected to be moderately volatile, affected by several important factors. These include technology developments, increased demand for cloud-based testing platforms, customized requirements, and regional pricing disparities. Furthermore, the growing adoption of AI and IoT technologies, combined with a greater emphasis on data security and regulation compliance, is putting a premium on the cost of in-vitro toxicity testing solutions.
Graph shows general upward trend pricing for In-Vitro Toxicology and growing demand. However, there may be fluctuations influenced by economic conditions, technological advancements, and competitive dynamic.
Efforts to simplify procurement procedures, strengthen supplier relationships, and implement modular toxicological testing platforms are crucial for cost containment. Using digital tools for market monitoring and predictive analytics for pricing forecasting and streamlining contract management can further enhance cost-efficiency.
Partnering with reputable in-vitro toxicology suppliers, negotiating multi-year contracts, and investigating subscription-based or usage-based pricing models are all good expense-management measures. Despite these issues, focusing on scalability, guaranteeing robust testing platform installation, and implementing cloud-based solutions will be critical for preserving cost-effectiveness and high-quality testing standards.
Cost Breakdown for In-Vitro Toxicology Total Cost of Ownership (TCO) and Cost-Saving Opportunities
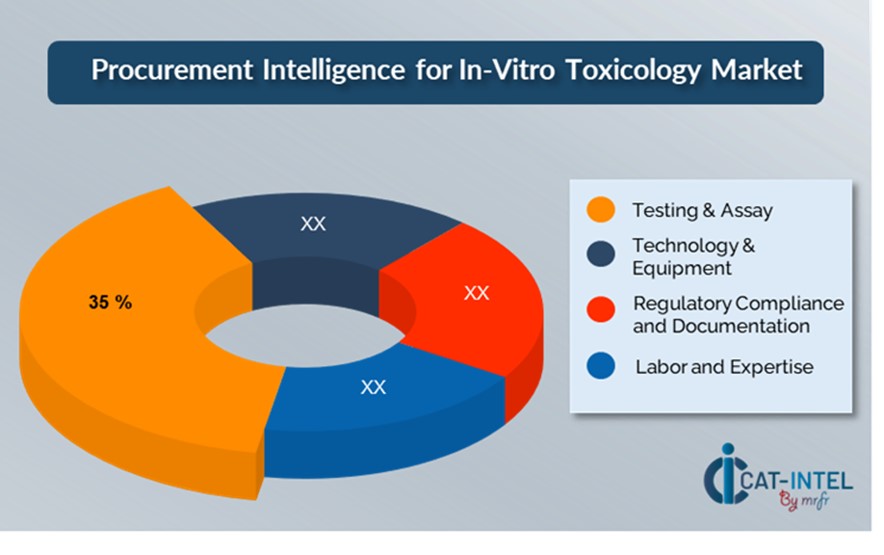
- Testing & Assay (35%)
-
Description This category includes the costs of performing various in-vitro toxicology tests and assays, such as cell-based assays, high-throughput screening, and specialist tests (for example, genotoxicity and cytotoxicity).
-
Trend There is an increasing tendency of using more cost-effective and standardized assays, such as automated high-throughput screening devices.
- Technology & Equipment (XX%)
- Regulatory Compliance and Documentation (XX%)
- Labour and Expertise (XX%)
Cost-Saving Opportunities Negotiation Levers and Purchasing Negotiation Strategies
In the in-vitro toxicology sector, streamlining procurement processes and using strategic bargaining strategies can result in significant cost savings and increased operational efficiency. Long-term relationships with reputable toxicology solution providers, particularly those that offer cloud-based platforms, can result in lower pricing and more attractive terms, including as volume-based discounts and bundled service packages. Subscription-based arrangements and multi-year contracts allow you to get reduced rates while mitigating the impact of price rises over time.
Collaborating with toxicology providers who value innovation and scalability provides additional benefits, like as access to advanced analytics, AI integration, and tailored testing solutions. These advancements can lower long-term operational expenses by reducing testing operations and improving predictive accuracy. Implementing digital procurement technologies, such as contract management platforms and usage analytics, increases transparency, reduces over-provisioning of resources, and optimizes the use of in-vitro testing platforms. Diversifying vendor alternatives and implementing multi-vendor methods can assist reduce reliance on a single source, mitigate risks like as service outages, and increase negotiation power, resulting in consistent access to high-quality toxicological solutions.
Supply and Demand Overview for In-Vitro Toxicology Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The in-vitro toxicology market is enjoying continuous expansion, led by increased efforts for digital transformation and innovations in testing, technologies used in pharmaceuticals, chemicals, and consumer goods. Technological advancements, industry-specific regulatory requirements, and global economic conditions all have an impact on supply and demand dynamics.
Demand Factors
-
Digital Transformation Initiatives In-vitro toxicology solutions are in high demand across sectors due to the increased requirement for centralized data administration, testing automation, and enhanced predictive analytics. -
Cloud Adoption Trends The shift to cloud-based toxicology platforms is driving up demand for scalable, subscription-based solutions that provide real-time data access and increased communication among research teams. -
Industry-Unique Requirements Pharmaceutical, chemical, and cosmetic industries demand in-vitro testing solutions that are adapted to regulatory standards and unique product safety requirements. -
Integration Capabilities There is an increasing demand for in vitro toxicology systems that can smoothly interact with other corporate applications, laboratory equipment, and IoT-enabled devices, facilitating better data management and analysis.
Supply Factors
-
Technological Advancements Advances in AI, machine learning, and cloud computing are improving the capabilities of in-vitro toxicology platforms, allowing for more accurate risk assessment, faster findings, and better predictive modelling -
Vendor Ecosystem An increasing number of in-vitro toxicology solution providers, that vary from niche players to enormous scale organizations, present businesses with a broad range of solutions according on their individual requirements. -
Global Economic Factors Exchange rates, labour costs, and regional technology adoption rates all have an impact on the pricing and availability of in-vitro testing platforms, with some locations benefiting from sophisticated infrastructure. -
Scalability and Flexibility Modern in-vitro toxicology platforms are increasingly modular, allowing suppliers to serve organizations of various kinds, from tiny research labs to huge multinational corporations.
Regional Demand-Supply Outlook In-Vitro Toxicology
The Image shows growing demand for In-Vitro Toxicology in both North America and Asia Pacific, with potential price increases and increased Competition.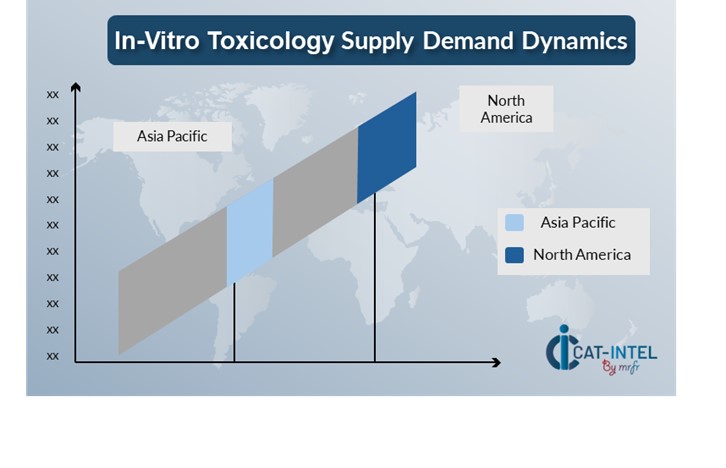
North America Dominance in the In-Vitro Toxicology Market
North America, particularly the United States, is a dominant force in the global In-Vitro Toxicology market due to several key factors
-
Advanced Regulatory Standard North America, notably the United States, has strict regulatory standards established by agencies such as the FDA (Food and Drug Administration) and the EPA (Environmental Protection Agency). -
Strong Pharmaceutical Industries Leading pharmaceutical, biotechnology, and chemical firms in North America rely on modern toxicity testing solutions for medication development, product safety, and environmental risk assessment. -
Technological Innovation North America is at the forefront of the development and implementation of cutting-edge technologies like artificial intelligence, machine learning, and high-throughput screening, all of which are progressively being integrated into in-vitro toxicity testing systems. -
Established Healthcare Infrastructure The region boasts a robust healthcare infrastructure, including research organizations, universities, and private laboratories specializing in toxicology.
-
Sustainable Testing Methods Regulatory entities, as well as public and commercial groups, promote the transition to in-vitro testing methods, which are regarded as more compassionate, cost-effective, and in line with environmental sustainability objectives.
North America Remains a key hub In-Vitro Toxicology Price Drivers Innovation and Growth.
Supplier Landscape Supplier Negotiations and Strategies
The supplier environment in the in-vitro toxicology market is diversified and highly competitive, with global leaders and regional players shaping industry dynamics. These vendors have an impact on critical elements like pricing, customisation, and service quality. Well-established suppliers dominate the market, offering full in-vitro testing platforms, while smaller, niche companies focus on specific sector demands or unique capabilities such as advanced analytics and AI-powered insights.
The in-vitro toxicology supplier ecosystem spans key technology regions, with both established global firms and creative local vendors meeting the regulatory and operational needs of many industries. As enterprises increasingly focus on digital transformation and improving safety standards, in-vitro toxicology suppliers are developing cloud-based solutions, incorporating emerging technologies such as AI and machine learning, and providing flexible subscription-based models to address the changing needs of pharmaceutical, chemical, and consumer goods firms. These developments are critical for increasing testing efficiency, accuracy, and compliance while maintaining scalability and cost-effectiveness in product safety evaluations.
Key Suppliers in the In-Vitro Toxicology Market Include
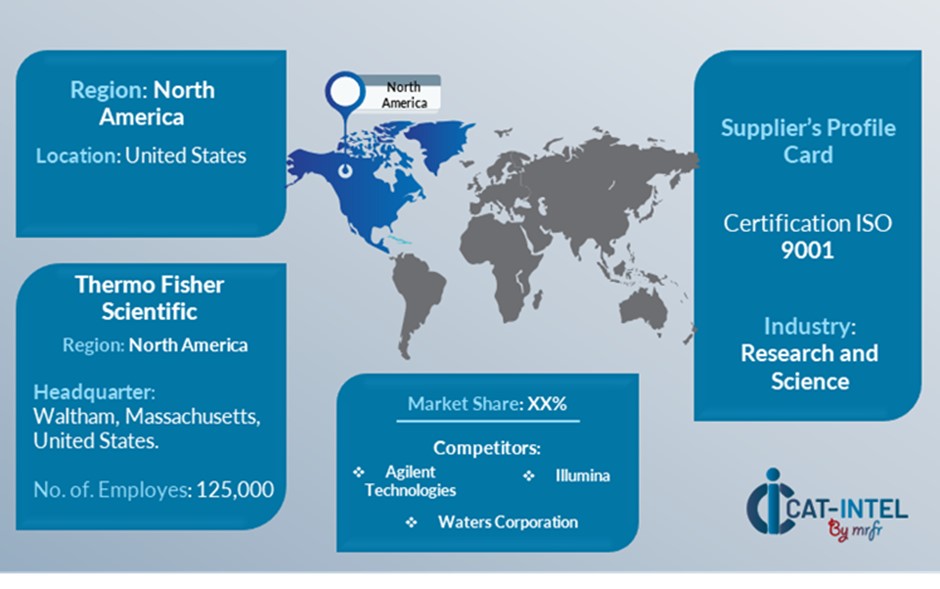
- Thermo Fisher Scientific
- Charles River Laboratories
- Lonza Group
- Eurofins Scientific
- Invitro International
- Bio Reliance
- Wuxi AppTec
- Toxiko
- JRC
- Kiwa Group
Key Developments Procurement Category Significant Development
Significant Development |
Description |
Market Growth |
Companies are using in-vitro approaches to speed safety evaluations, minimize reliance on animal testing, and improve regulatory compliance, particularly in emerging regions where regulatory frameworks are fast changing. |
Cloud Adoption |
As research teams operate from many locations and hybrid work models grow more prevalent, cloud-based solutions enable seamless collaboration, real-time data sharing, and efficient managing resources. |
Product Innovation |
In-vitro toxicology companies are increasing their solutions by including AI-powered analytics, real-time data processing, and industry-specific testing modules customized for sectors like pharmaceuticals, chemicals, and consumers goods. |
Technological Advancements |
Technological improvements like as machine learning, IoT integration, and robotic process automation (RPA) are improving in-vitro toxicological capabilities, allowing for more efficient and reliable safety assessments. |
Global Trade Dynamics |
Multinational corporations with complicated supply chains are increasingly relying on these solutions to comply with varied international requirements and provide uniformity in product safety testing across areas. |
Customization Trends |
Companies are looking for modular platforms that can be configured to interact with third-party tools, adapt to changing regulatory needs, and support a variety of testing procedures, providing greater flexibility and tailored solutions. |
In-Vitro Toxicology Attribute/Metric |
Details |
Market Sizing |
The global In-Vitro Toxicology market is projected to reach USD 49.06 billion by 2035, growing at a CAGR of approximately 11.22% from 2025 to 2035. |
In-Vitro Toxicology Technology Adoption Rate |
Around 60% of enterprises in areas such as pharmaceuticals, chemicals, and cosmetics have used in-vitro testing systems, with a noticeable move toward cloud-based platforms. |
Top In-Vitro Toxicology Industry Strategies for 2025 |
Key strategies include incorporating AI and machine learning for predicting toxicity evaluations, simplifying safety evaluation processes, modular testing systems that prioritize sustainability by decreasing animal testing and improving data security to meet regulatory requirements.
|
In-Vitro Toxicology Process Automation |
Approximately half of in-vitro toxicology platforms automate common operations including toxicity screening, data processing, and reporting, increasing the efficiency of safety evaluations.
|
In-Vitro Toxicology Process Challenges |
High testing costs, integrating new technologies into current systems, regulatory compliance, and assuring data accuracy across several testing methodologies are all significant problems.
|
Key Suppliers |
Leading in-vitro toxicology solution providers include Thermo Fisher Scientific (USA), Charles River Laboratories (USA), and L'Oréal Research and Innovation (France), who provide innovative solutions across a variety of sectors.
|
Key Regions Covered |
North America, Europe, and Asia-Pacific are key markets for in-vitro toxicology adoption, with considerable demand from the pharmaceutical, chemical, and cosmetics industries.
|
Market Drivers and Trends |
The growing demand for safer and more efficient testing methods, the rise of regulations for product safety, the adoption of cloud-based services for real-time data availability, and the incorporation of AI and machine learning for better predictive analytics and automation are all driving expansion. |










