Summary Overview
High Potency Drug Manufacturing Market Overview
The global high-potency drug manufacturing market is expanding rapidly, fuelled by rising demand in key industries such as oncology, neurology, and specialist therapies. This market encompasses a wide range of production methods, including novel drug formulation techniques, enhanced containment systems, and cutting-edge analytical processes. Our paper provides a detailed examination of production trends, with an emphasis on cost-cutting tactics, improved safety protocols, and the use of new technology to simplify manufacturing operations.
Key future problems in high-potency drug manufacturing include cost management, scalability, severe safety and regulatory compliance, and integrating new technology into existing production systems. The use of automated manufacturing equipment, innovative containment systems, and effective supply chain management is crucial for optimizing medication production and maintaining long-term market competitiveness.
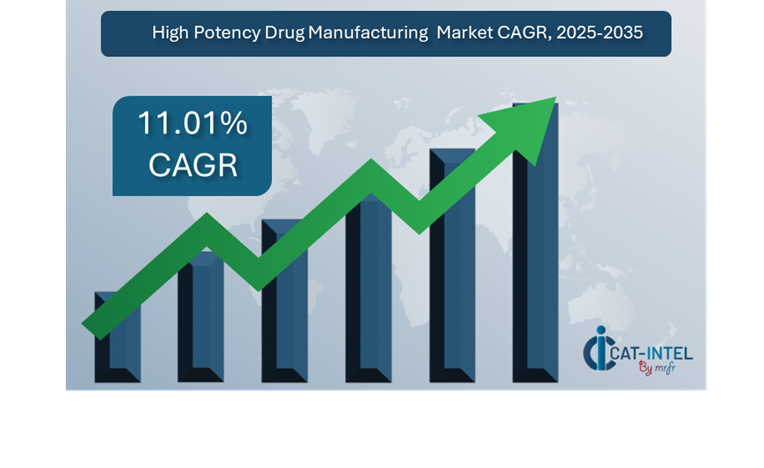
Market Size: The global High Potency Drug Manufacturing market is projected to reach USD 615.6 billion by 2035, growing at a CAGR of approximately 11.01% from 2025 to 2035.
-
Sector Contributions: Growth in the market is driven by: -
Manufacturing and Supply Chain Optimization: Demand for real-time data and process integration in the high-potency drug production sector is growing, driven by the desire for more efficient operations.
-
Pharmaceutical Growth and Innovation: As the pharmaceutical industry expands, particularly in specialised and high-potency medications, producers are being pushed to embrace novel production techniques and improve safety measures.
-
Technological Advancements: AI, machine learning, and automation are transforming the high-potency medication manufacturing process.
-
Innovative Manufacturing Solutions: The transition to modular and flexible manufacturing solutions enables pharmaceutical businesses to choose certain technologies or processes that meet their specific requirements.
-
Investment Initiatives: Pharmaceutical businesses are heavily investing in sophisticated production technology, including as cloud-based platforms that provide real-time monitoring and remote access.
-
Regional Insights: North America and Asia Pacific are developing as significant hubs for high-potency drug manufacturing due to improved digital infrastructure and rising demand for specialised drugs.
Key Trends and Sustainability Outlook:
-
Cloud Integration: Improves scalability, lowers infrastructure costs, and gives you better access to current information for making choices and process optimization.
-
Advanced Features: The integration of new technologies such as AI, IoT, and blockchain improves transparency, allows for improved decision-making, and automates production processes.
-
Focus on Sustainability: Pharmaceutical companies are putting more emphasis on sustainability, adopting modern technologies to manage resource usage, reduce waste, and meet regulatory sustainability targets.
-
Customization Trends: Customization enables businesses to handle specific industry concerns, such as safety and precision, while reducing risk.
-
Data-Driven Insights: Using advanced analytics enables pharmaceutical manufactures to optimize production schedules, manage inventory efficiently.
Growth Drivers:
-
Digital Transformation: Pharmaceutical businesses are adopting digital transformation to improve production efficiency, collaborate more effectively, and maintain the flexibility required to adapt to rapidly changing industry demands.
-
Demand for Process Automation: The pharmaceutical sector is rapidly turning to automation to manage repetitive processes, reduce human error, and improve manufacturing safety, particularly in high-potency medication manufacture.
-
Scalability Requirements: pharmaceutical businesses are investing in scalable manufacturing systems that can smoothly interact with existing operations while meeting rising output demands.\
-
Regulatory Compliance: High-potency medication makers must follow tight regulatory guidelines, and automation is critical to assuring compliance.
-
Globalization: The need for high-potency medications is global, prompting pharmaceutical corporations to explore solutions that enable multi-regional manufacture and compliance with international regulatory norms.
Overview of Market Intelligence Services for the High Potency Drug Manufacturing Market:
Recent studies have highlighted substantial hurdles in the high-potency drug manufacturing business, including high production costs and the need for process customisation to fulfil specific safety and regulatory criteria. Market intelligence studies give valuable information that allow businesses to find cost-cutting opportunities, optimize supplier relationships, and ensure seamless scaling of production processes. These insights also contribute to ensuring compliance with industry rules, maintaining high-quality production standards, and successfully managing operating expenses in a highly regulated environment.
Procurement Intelligence for High Potency Drug Manufacturing: Category Management and Strategic Sourcing
To remain competitive in the high-potency medicine manufacturing sector, businesses are improving procurement processes through strategic expenditure analysis and supplier assessment of performance. Effective category management and strategic sourcing are critical for lowering procurement costs and ensuring a steady supply of high-quality materials and technologies for manufacturing. Pharmaceutical firms may improve their procurement strategy, negotiate better contracts, and find specialized equipment and raw materials at reasonable costs by employing actionable market intelligence. This technique ensures a dependable supply chain, lowers operational risks, and facilitates the effective scaling of high-potency medication production.
Pricing Outlook for High Potency Drug Manufacturing: Spend Analysis
The price prognosis for high-potency drug manufacturing technologies and solutions is projected to remain moderately volatile, impacted by several major factors. These include technological developments, increased need for specialized production systems, customization requirements to satisfy specific regulatory standards, and regional pricing discrepancies. Furthermore, the increasing use of automation, AI, IoT, and a greater emphasis on data security and regulatory compliance are raising costs, particularly for high-end manufacturing equipment and technology.
Graph shows general upward trend pricing for High Potency Drug Manufacturing and growing demand. However, there may be fluctuations influenced by economic conditions, technological advancements, and competitive dynamic.
Efforts to streamline procurement procedures, enhance vendor management, and explore flexible, modular manufacturing methods are crucial for minimizing costs in this industry. Leveraging digital technologies for market monitoring, predictive pricing forecasting, and improved contract management can help to enhance cost efficiency and assure long-term financial viability.
Partnering with trustworthy suppliers, negotiating multi-year contracts, and exploring subscription-based or pay-per-use models for specialized production technologies are all excellent cost-cutting techniques. While there are hurdles, focusing on scalability, developing a strong manufacturing implementation plan, and using cloud-based platforms for real-time monitoring will be critical to attaining cost-effectiveness while fulfilling the growing demand for high-potency pharmaceuticals.
Cost Breakdown for High Potency Drug Manufacturing: Total Cost of Ownership
(TCO) and Cost-Saving Opportunities
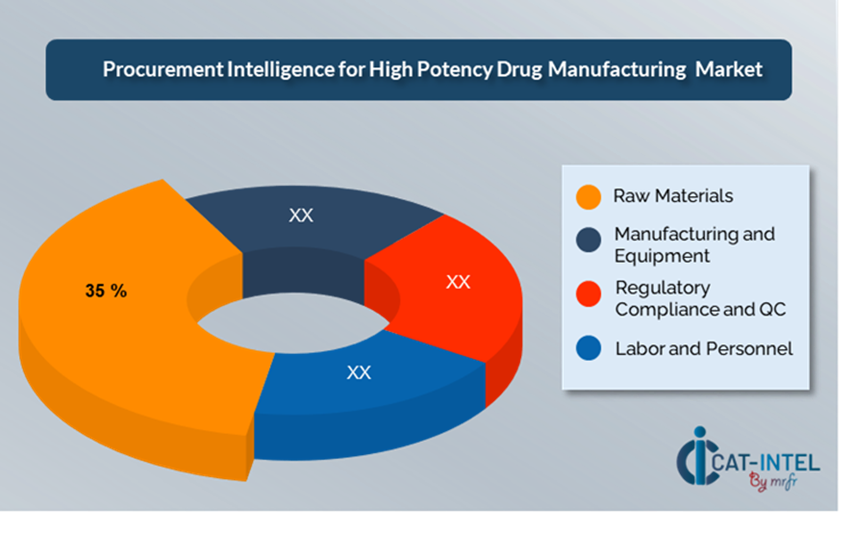
- Raw Material: (35%)
-
Description: This cover active pharmaceutical ingredients (APIs), excipients, solvents, and other chemical substances needed in manufacture.
-
Trends: The demand for specialized and superior raw materials, especially for biologics and complicated compounds, is increasing.
- Manufacturing and Equipment: (XX%)
- Regulatory Compliance and QC: (XX%)
- Labour and Personnel: (XX%)
Cost-Saving Opportunities: Negotiation Levers and Purchasing Negotiation Strategies
In the high-potency medicine manufacturing business, improving procurement processes and using strategic bargaining strategies can result in significant cost reductions and improved operational efficiency. Establishing long-term Agreements with providers of vital production technologies, particularly those that provide scalable, cloud-based solutions, can lead to more attractive pricing arrangements, such as volume discounts and bundled service packages. Subscription-based arrangements and multi-year contracts allow you to lock in reduced rates while reducing the impact of price rises over time.Collaborating with suppliers who promote innovation and scalability provides additional benefits, such as access to cutting-edge technology like AI for predictive analytics, IoT for increased monitoring, and modular solutions adapted to specific manufacturing requirements. This not only helps to decrease long-term operational expenses but also ensures that manufacturing processes are adaptable to changing needs and regulatory requirements. Implementing digital technologies for procurement, such as contract management systems and use analytics, can promote transparency, reduce over-provisioning of resources, and optimize the utilization of high-potency manufacturing equipment.
Supply and Demand Overview for High Potency Drug Manufacturing: Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The high-potency drug manufacturing market is expanding steadily, propelled by increased digital transformation activities in the pharmaceutical and healthcare sectors. Technological improvements, regulatory regulations, and global economic situations all have an impact on supply and demand in this market.
Demand Factors:
-
Digital Transformation Initiatives: Companies are increasingly turning to digital technologies to streamline production processes, eliminate human error, and improve quality control, particularly in high-potency medicine manufacturing.
-
Cloud Adoption Trends: A transition to cloud-based technologies is transforming the manufacturing landscape, allowing pharmaceutical businesses to streamline supply chains, measure production data, and manage inventory more effectively.
-
Industry-Specific Guidelines: The pharmaceutical industry, particularly high-potency medication manufacture, requires customized solutions that adhere to tight regulatory standards such as FDA guidelines and Good Manufacturing Practices (GMP).
-
Integration Capabilities: There is an increasing demand for manufacturing systems that work smoothly with other corporate software, such as management of supply chains, quality control tools, and Internet of Things devices.
Supply Factors:
-
Technological Advancements: Advancements in AI, machine learning, and cloud computing are propelling the creation of increasingly modern manufacturing technologies. Making suppliers more competitive by providing innovative solutions.
-
Vendor Ecosystem: The growing number of suppliers, which range from huge multinational corporations to boutique technology providers, is generating a diversified market for high-potency drug production solutions.
-
Global Economic Factors: Exchange rates, labour costs, and regional technological adoption rates, can have a substantial impact on manufacturing technology pricing and availability.
-
Scalability and Flexibility: Modern manufacturing processes are becoming increasingly modular, allowing vendors to offer flexible systems that cater to organizations of different sizes and complexities.
Regional Demand-Supply Outlook: High Potency Drug Manufacturing
The Image shows growing demand for High Potency Drug Manufacturing in both North America and Asia Pacific, with potential price increases and increased Competition.
North America: Dominance in the High Potency Drug Manufacturing Market
North America, particularly the United States, is a dominant force in the global High Potency Drug Manufacturing market due to
-
Advanced Healthcare Infrastructure: The region is a leader in drug innovation, particularly in high-potency formulations, thanks to significant investments in pharmaceutical R&D, which require high-potency manufacturing processes.
-
Stringent Regulatory Standards: North America's regulatory standards, enforced by the FDA and Health Canada, are among the strictest globally.
-
Strong Pharmaceutical Industries:North America has a strong pharmaceutical and biotechnology industry presence, with many major worldwide corporations. Their large-scale industrial facilities and cooperation help the area maintain its dominance.
-
Skilled Workforce and Technology: North America's skilled workforce and innovative manufacturing technology, including as automated procedures, containment systems, and cutting-edge equipment, enable the safe and efficient production of high-potency pharmaceuticals.
-
Market Dynamics and Demand: The market for high-potency medications is quickly developing, driven by an aging population and rising prevalence of chronic diseases like cancer.
North America Remains a key hub High Potency Drug Manufacturing Price Drivers Innovation and Growth.
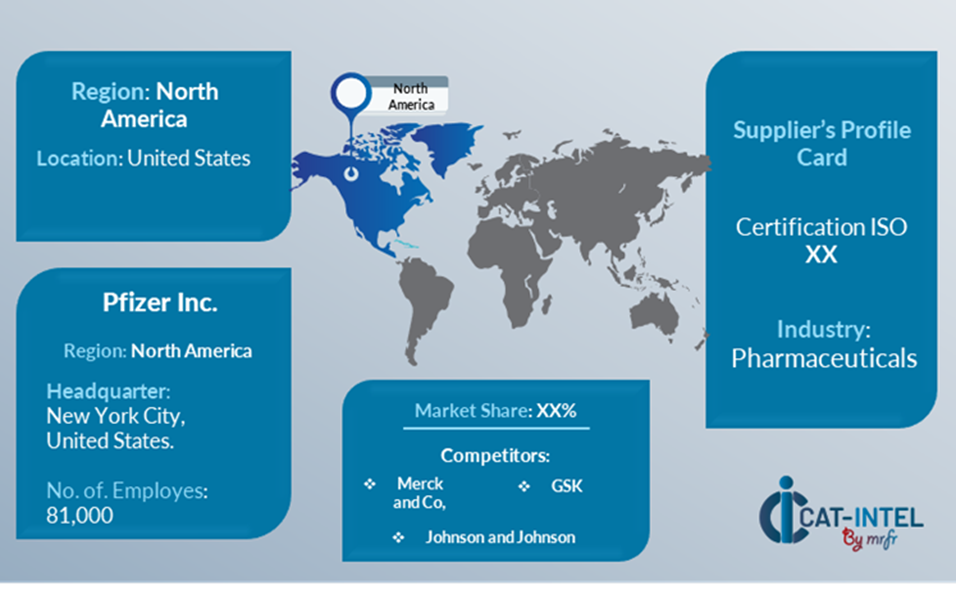
Supplier Landscape: Supplier Negotiations and Strategies
The supplier environment in the high-potency drug manufacturing sector is similarly broad and competitive, with a mix of global market leaders and regional players. These vendors have a significant impact on essential elements like price models, customization options, regulatory compliance, and service quality. Established pharmaceutical technology providers dominate the industry by providing entire production systems, while smaller, specialized suppliers focus on specialty requirements such as high-potency confinement, safety features, and sophisticated analytics for process optimization.
The high-potency drug manufacturing market is distinguished by the presence of major global suppliers that provide large-scale production systems, as well as regional players that cater to specific needs, such as tailored safety protocols, compliance with local regulations, and customized high-potency drug production techniques. As digital transformation and the desire for efficiency continue to alter the pharmaceutical sector, suppliers are focusing more on expanding their technology offerings and adapting solutions to satisfy the complicated needs of high-potency drug producers.
Key Suppliers in the High Potency Drug Manufacturing Market Include:
- Pfizer Inc.
- Lonza Group
- Boehringer Ingelheim
- WuXi AppTec
- Cipla Ltd.
- Cambrex Corporation
- Sandoz
- Patheon
- Aenova Group
- Hovione
Key Developments Procurement Category Significant Development:
Significant Development |
Description |
Market Growth |
Companies are rapidly using digital technologies and automation to optimize operations, increase efficiency, and assure strict regulatory compliance, particularly in expanding pharmaceutical markets. |
Cloud Adoption |
Cloud solutions provide scalability, remote monitoring, and improved data accessibility, which are critical for managing the intricacies of global manufacturing processes while meeting regulatory standards. |
Product Innovation |
Suppliers in the high-potency medicine production industry are increasingly incorporating new technology into their products. AI-powered analytics and real-time data processing are boosting manufacturing line efficiency and safety measures. |
Technological Advancements |
Machine learning, IoT integration, and robotic process automation (RPA) are changing the manufacturing of high-potency drugs. Machine learning and artificial intelligence are being used to predict equipment failures, streamline supply networks, and enhance medicine quality control. |
Global Trade Dynamics |
Changes in global trade restrictions, compliance standards, and regional economic policies have an impact on the adoption of innovative manufacturing technology. Multinational pharmaceutical companies must change their manufacturing operations to meet varied regulatory regimes in different locations. |
Customization Trends |
Integration with independent tools and technologies, such as quality control systems (QMS) or track-and-trace solutions, is becoming increasingly vital for ensuring smooth operations, improved traceability, and compliance with industry standards. |
|
High Potency Drug Manufacturing Attribute/Metric |
Details |
Market Sizing |
The global High Potency Drug Manufacturing market is projected to reach USD 615.6 billion by 2035, growing at a CAGR of approximately 11.01% from 2025 to 2035.
|
High Potency Drug Manufacturing Technology Adoption Rate |
Around 55% of pharmaceutical firms have implemented advanced digital solutions, with a strong preference for cloud-based platforms for increased scalability, real-time data monitoring, and remote access to production data. |
Top High Potency Drug Manufacturing Industry Strategies for 2025 |
Key methods include using artificial intelligence for predictive maintenance, optimizing manufacturing operations with modular systems, emphasizing cybersecurity for critical production data, and implementing mobile platforms for real-time monitoring and collaboration.
|
High Potency Drug Manufacturing Process Automation |
Approximately 50% of high-potency drug production facilities have automated routine operations including batch control, inventory management, and compliance reporting in order to improve operational efficiency and eliminate human error.
|
High Potency Drug Manufacturing Process Challenges |
Major hurdles include high upfront investment costs, reluctance to change in manufacturing teams, equipment calibration issues, data migration from outdated systems, and the requirement for regular system updates and maintenance to assure compliance.
|
Key Suppliers |
Sartorius, Rockwell Automation, Siemens Healthineers, and Honeywell Process Solutions are among the top high-potency drug manufacturing vendors, offering specialized equipment for containment, automation, and real-time monitoring.
|
Key Regions Covered |
North America, Europe, and Asia-Pacific are key locations for the adoption of sophisticated technologies in high-potency drug manufacturing, with major demand in pharmaceutical hubs such as the United States, Germany, Switzerland, India, and China.
|
Market Drivers and Trends |
Growth factors include rising demand for high-potency pharmaceuticals, greater regulatory compliance, increased use of automation and AI in manufacturing, a growing requirement for real-time data and predictive analytics, and the incorporation of IoT for improved process monitoring and quality control. |










