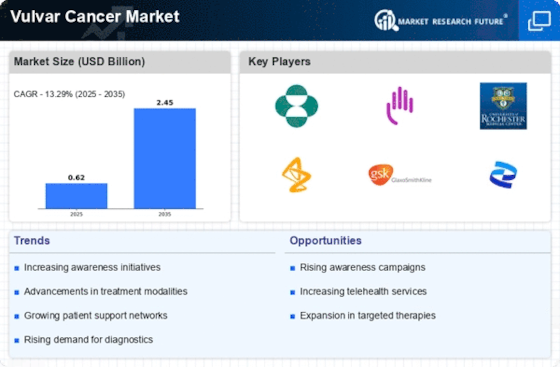
Top Industry Leaders in the Vulvar Cancer Market

Roche Holding AG Received FDA approval for their FoundationOne CDx assay, a comprehensive genomic profiling test, to guide personalized treatment decisions in vulvar cancer patients.
Guardant Health Expanded their liquid biopsy testing platform to include vulvar cancer, allowing for non-invasive tumor profiling and potentially improving treatment planning.
Myriad Genetics Developed a new genetic test panel specifically for vulvar cancer, offering a more comprehensive analysis of potential genetic risk factors and personalized treatment options.
Boston Scientific Corporation Launched a new vaginal dilator specifically designed for vulvar cancer survivors, addressing a critical need for post-treatment recovery and intimacy support.
Hologic Introduced their Aptima® HPV test with improved sensitivity for detecting HPV strains associated with vulvar cancer, contributing to earlier diagnosis and prevention efforts.
List of Vulvar Cancer Key Companies in the Market
- Amgen Inc. (US)
- Bristol-Myers Squibb Company (US)
- Celgene Corporation (US)
- Eli Lilly and Company (US)
- Hoffmann-La Roche Ltd (Switzerland)
- Merck & Co.Inc. (US)
- Novartis AG (Switzerland)
- Pfizer Inc. (US)
- GlaxoSmithKline plc (UK)
- Sanofi (France)
- and Johnson & Johnson Services Inc. (US)