Enhanced Patient Recruitment
The Virtual Clinical Trials Market is experiencing a notable shift in patient recruitment strategies. Traditional methods often face challenges such as geographical limitations and low participation rates. However, virtual trials leverage digital platforms to reach a broader audience, potentially increasing enrollment by up to 30%. This enhanced recruitment capability not only accelerates trial timelines but also diversifies participant demographics, which is crucial for the validity of clinical outcomes. As a result, pharmaceutical companies are increasingly adopting virtual methodologies to streamline their recruitment processes, thereby driving growth in the Virtual Clinical Trials Market.
Regulatory Advancements and Support
Regulatory bodies are increasingly recognizing the potential of virtual trials, which is influencing the Virtual Clinical Trials Market. Recent guidelines have been established to facilitate the integration of digital technologies in clinical research. These advancements not only provide a framework for compliance but also encourage innovation in trial designs. As regulatory support continues to evolve, it is expected that more organizations will embrace virtual methodologies, thereby enhancing the overall efficiency and effectiveness of clinical trials. This trend indicates a promising future for the Virtual Clinical Trials Market.
Technological Integration and Innovation
The integration of advanced technologies is a driving force in the Virtual Clinical Trials Market. Innovations such as artificial intelligence, wearable devices, and telemedicine are transforming how trials are conducted. These technologies enable real-time data collection and monitoring, enhancing patient safety and data integrity. Furthermore, the use of mobile applications for patient engagement is becoming increasingly prevalent, allowing for seamless communication between participants and researchers. As these technologies continue to evolve, they are likely to further streamline trial processes, thereby fostering growth in the Virtual Clinical Trials Market.
Cost Efficiency and Resource Optimization
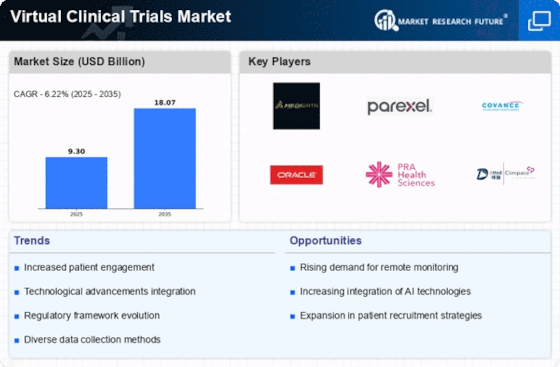
Cost considerations play a pivotal role in the Virtual Clinical Trials Market. By utilizing virtual platforms, organizations can significantly reduce operational costs associated with traditional trials, such as site management and patient travel expenses. Reports indicate that virtual trials can lower costs by approximately 20 to 30%. This financial advantage allows sponsors to allocate resources more effectively, potentially leading to increased investment in innovative trial designs. Consequently, the emphasis on cost efficiency is likely to propel the adoption of virtual trials, further expanding the Virtual Clinical Trials Market.
Increased Focus on Patient-Centric Approaches
The shift towards patient-centricity is reshaping the Virtual Clinical Trials Market. Stakeholders are increasingly prioritizing the needs and preferences of patients, which is reflected in the design and execution of clinical trials. Virtual trials offer greater flexibility and convenience for participants, allowing them to engage in research from the comfort of their homes. This focus on patient experience not only enhances recruitment and retention rates but also improves data quality. As the industry continues to embrace patient-centric models, the demand for virtual trials is expected to rise, further propelling the Virtual Clinical Trials Market.