Growing Demand for Gene Therapies
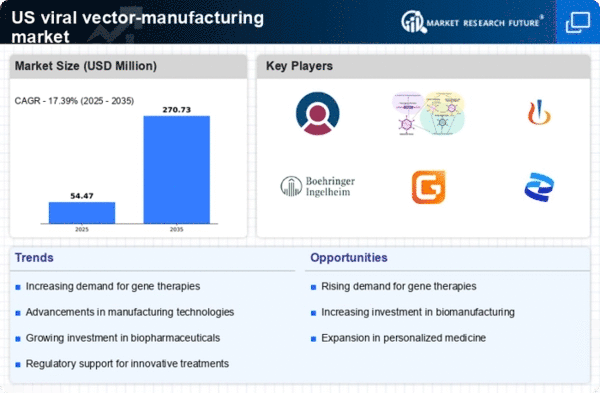
The viral vector-manufacturing market is experiencing a surge in demand for gene therapies, driven by the increasing prevalence of genetic disorders and chronic diseases. As of 2025, the market for gene therapies is projected to reach approximately $10 billion in the US, indicating a robust growth trajectory. This demand is further fueled by advancements in genetic research and the successful outcomes of clinical trials, which have demonstrated the efficacy of viral vectors in delivering therapeutic genes. Consequently, manufacturers are compelled to enhance their production capabilities to meet this escalating demand, thereby propelling the growth of the viral vector-manufacturing market.
Rising Investment in Biopharmaceuticals
The viral vector-manufacturing market is witnessing a notable increase in investment within the biopharmaceutical sector. As companies recognize the potential of viral vectors in drug development, funding for research and production capabilities is on the rise. In 2025, investments in biopharmaceuticals are projected to exceed $50 billion in the US, with a significant portion allocated to viral vector technologies. This influx of capital is likely to drive innovation and expand production capacity, thereby strengthening the overall landscape of the viral vector-manufacturing market.
Regulatory Support for Advanced Therapies
Regulatory bodies in the US are providing increased support for the development of advanced therapies, which is positively influencing the viral vector-manufacturing market. Initiatives aimed at expediting the approval process for gene therapies and other advanced treatments are encouraging manufacturers to invest in viral vector production. The FDA's commitment to fostering innovation in this sector is evident through various programs designed to streamline regulatory pathways. This supportive environment is expected to enhance the growth prospects of the viral vector-manufacturing market, as more therapies gain approval and enter the market.
Technological Advancements in Vector Production
Technological innovations in the production of viral vectors are significantly impacting the viral vector-manufacturing market. The introduction of novel bioprocessing techniques, such as suspension cell culture and continuous manufacturing, has improved yield and efficiency. These advancements not only reduce production costs but also enhance the scalability of viral vector production. As a result, manufacturers are increasingly adopting these technologies to streamline their operations. The market is expected to witness a compound annual growth rate (CAGR) of around 15% over the next five years, reflecting the positive influence of these technological advancements on the viral vector-manufacturing market.
Increased Collaboration Between Academia and Industry
The viral vector-manufacturing market is benefiting from heightened collaboration between academic institutions and industry players. This synergy fosters innovation and accelerates the translation of research findings into practical applications. Numerous partnerships have emerged, focusing on the development of novel viral vectors and their applications in therapeutics. Such collaborations not only enhance the research capabilities of manufacturers but also facilitate access to cutting-edge technologies. As a result, the viral vector-manufacturing market is likely to expand, with an increasing number of products entering clinical trials and subsequent commercialization.