Rising Incidence of Spina Bifida
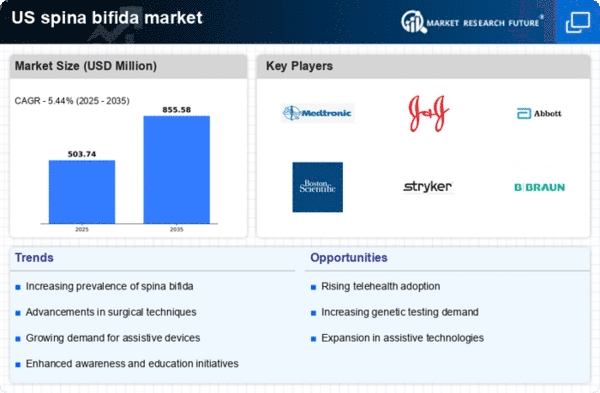
The spina bifida market is experiencing growth due to the rising incidence of this condition in the United States. Recent data indicates that approximately 1,500 to 2,000 babies are born with spina bifida annually, which translates to a prevalence rate of about 3 per 1,000 live births. This increasing number of cases necessitates enhanced medical interventions and support services, thereby driving demand within the spina bifida market. Furthermore, the growing awareness among healthcare providers and parents regarding the importance of early diagnosis and treatment options is likely to contribute to market expansion. As more families seek specialized care, the spina bifida market is poised to benefit from the heightened need for surgical procedures, rehabilitation services, and assistive technologies.
Government Initiatives and Funding
Government initiatives and funding programs are significantly influencing the spina bifida market. Various federal and state programs aim to support research, treatment, and education related to spina bifida. For instance, the National Institutes of Health (NIH) allocates substantial resources for research aimed at understanding the causes and potential treatments for spina bifida. Additionally, funding for public health campaigns to raise awareness about the condition is likely to enhance early detection and intervention efforts. These initiatives not only improve patient outcomes but also stimulate the spina bifida market by increasing the availability of resources and support services. As government involvement continues to grow, the market may witness a positive impact on accessibility and affordability of care.
Rising Demand for Assistive Devices
The spina bifida market is witnessing a rising demand for assistive devices that enhance mobility and independence for affected individuals. As awareness of the condition grows, families are increasingly seeking solutions that can improve the quality of life for their children. Assistive technologies, such as wheelchairs, braces, and adaptive equipment, are becoming essential components of care for individuals with spina bifida. The market for these devices is projected to expand as innovations in design and functionality continue to emerge. Additionally, the increasing availability of funding and insurance coverage for assistive devices is likely to further stimulate demand. As a result, the spina bifida market may experience significant growth driven by the need for effective and accessible assistive solutions.
Technological Innovations in Treatment
Technological advancements are playing a pivotal role in shaping the spina bifida market. Innovations in surgical techniques, such as in utero repair, have shown promising results in improving outcomes for affected infants. These procedures, which can be performed before birth, have the potential to reduce the severity of the condition and improve the quality of life for patients. Additionally, the development of advanced imaging technologies and minimally invasive surgical tools is enhancing the precision of interventions. The spina bifida market is likely to see increased investment in research and development as healthcare providers strive to adopt these cutting-edge technologies. As a result, the market may experience a surge in demand for specialized surgical centers and trained professionals, further driving growth.
Increased Focus on Pediatric Healthcare
The spina bifida market is benefiting from an increased focus on pediatric healthcare in the United States. As healthcare systems prioritize the needs of children with chronic conditions, there is a growing emphasis on specialized care for conditions like spina bifida. This shift is reflected in the establishment of dedicated pediatric clinics and multidisciplinary care teams that address the unique challenges faced by these patients. Furthermore, the rising prevalence of childhood disabilities is prompting healthcare providers to enhance their services, which is likely to drive demand within the spina bifida market. The integration of comprehensive care models that include physical therapy, occupational therapy, and psychological support is expected to improve patient outcomes and satisfaction.