Growing Aging Population
The increasing aging population in the US is a pivotal driver for the pressure relief-device market. As individuals age, they often experience a higher risk of pressure ulcers and related complications, necessitating the use of effective pressure relief devices. According to recent statistics, approximately 15% of the US population is aged 65 and older, a figure projected to rise significantly in the coming years. This demographic shift is likely to escalate the demand for pressure relief devices, as healthcare providers seek to mitigate the risks associated with prolonged immobility. Consequently, the pressure relief-device market industry is expected to witness substantial growth, with an anticipated increase in product innovation aimed at enhancing patient comfort and safety. The focus on preventive care in geriatric health further underscores the importance of these devices in clinical settings.
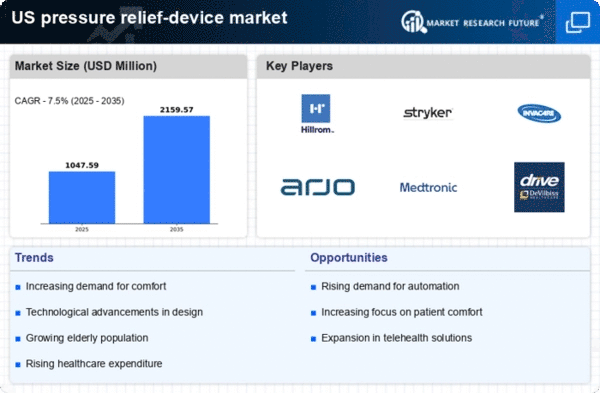
Rising Healthcare Expenditure
The upward trend in healthcare expenditure in the US serves as a crucial driver for the pressure relief-device market. With healthcare spending projected to reach $6 trillion by 2027, there is a growing emphasis on investing in preventive measures, including pressure relief devices. Hospitals and healthcare facilities are increasingly allocating budgets towards advanced medical equipment that can improve patient outcomes and reduce the incidence of pressure ulcers. This financial commitment indicates a shift towards prioritizing patient care, which is likely to bolster the pressure relief-device market industry. Furthermore, as reimbursement policies evolve to favor preventive care, healthcare providers may be more inclined to adopt innovative pressure relief solutions, thereby enhancing market growth. The integration of these devices into standard care protocols is expected to become more prevalent as healthcare costs continue to rise.
Regulatory Support for Quality Standards
Regulatory support for quality standards in healthcare is a vital driver for the pressure relief-device market. Agencies such as the FDA have established guidelines that ensure the safety and efficacy of medical devices, including those designed for pressure relief. Compliance with these regulations not only enhances product credibility but also encourages manufacturers to innovate and improve their offerings. The pressure relief-device market industry is likely to benefit from this regulatory framework, as it fosters a competitive environment where quality and performance are prioritized. Furthermore, adherence to stringent quality standards can lead to increased market access for manufacturers, as healthcare facilities seek to procure devices that meet regulatory requirements. This regulatory landscape is expected to stimulate growth in the market, as stakeholders recognize the importance of investing in high-quality pressure relief solutions.
Technological Innovations in Device Design
Technological innovations play a significant role in shaping the pressure relief-device market. The introduction of smart technologies, such as sensors and automated pressure adjustments, enhances the functionality and effectiveness of these devices. Recent advancements have led to the development of pressure relief systems that can monitor patient movements and adjust accordingly, thereby reducing the risk of pressure ulcers. The pressure relief-device market industry is witnessing a surge in demand for such innovative solutions, as healthcare providers seek to improve patient care and outcomes. Moreover, the integration of data analytics into device functionality allows for better tracking of patient health metrics, which can inform treatment decisions. As technology continues to evolve, the market is likely to see an influx of new products that cater to the specific needs of patients, further driving growth in this sector.
Increased Awareness of Pressure Ulcer Prevention
The heightened awareness surrounding pressure ulcer prevention is a significant driver for the pressure relief-device market. Educational campaigns and initiatives by healthcare organizations have led to a greater understanding of the risks associated with pressure ulcers, particularly among vulnerable populations. This awareness has prompted healthcare providers to adopt preventive measures, including the use of pressure relief devices, to enhance patient safety. The pressure relief-device market industry is benefiting from this shift in focus, as more facilities implement protocols aimed at reducing the incidence of pressure ulcers. Additionally, patient advocacy groups are actively promoting the importance of pressure relief solutions, further influencing healthcare practices. As awareness continues to grow, the demand for effective pressure relief devices is expected to rise, contributing to the overall expansion of the market.