Rising Prevalence of Diabetes
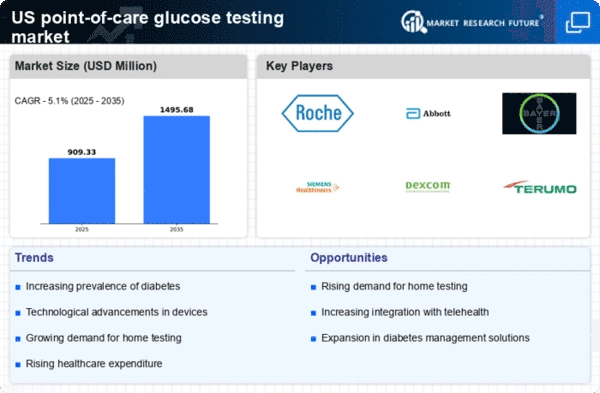
The increasing prevalence of diabetes in the US is a primary driver for the point of-care-glucose-testing market. According to the Centers for Disease Control and Prevention (CDC), approximately 34.2 million Americans, or 10.5% of the population, have diabetes. This growing patient population necessitates more accessible and efficient glucose monitoring solutions. As healthcare providers seek to improve patient outcomes, the demand for point-of-care testing devices is likely to rise. These devices enable rapid glucose level assessments, facilitating timely clinical decisions. Furthermore, the trend towards personalized medicine emphasizes the need for continuous glucose monitoring, which could further propel the market. The point-of-care-glucose-testing market is thus positioned to expand significantly as healthcare systems adapt to the increasing burden of diabetes.
Supportive Reimbursement Policies
Supportive reimbursement policies are playing a crucial role in the growth of the point-of-care-glucose-testing market. Insurance coverage for point-of-care testing devices is becoming more prevalent, which encourages healthcare providers to adopt these technologies. The Centers for Medicare & Medicaid Services (CMS) has recognized the importance of glucose monitoring in managing diabetes, leading to favorable reimbursement rates for point-of-care testing. This financial support reduces the burden on patients and healthcare providers, making it easier to implement these testing solutions. As reimbursement policies continue to evolve, they are likely to further stimulate the point-of-care-glucose-testing market, ensuring that more patients have access to essential monitoring tools.
Increased Focus on Preventive Healthcare
The shift towards preventive healthcare in the US is influencing the point-of-care-glucose-testing market. With rising healthcare costs and a growing emphasis on early disease detection, healthcare providers are increasingly adopting point-of-care testing solutions. These devices allow for immediate glucose level assessments, enabling timely interventions that can prevent complications associated with diabetes. The American Diabetes Association advocates for regular monitoring, which aligns with the preventive healthcare model. As a result, the point-of-care-glucose-testing market is expected to experience growth, driven by the need for efficient and effective monitoring solutions. The convenience and speed of these tests are appealing to both patients and healthcare providers, potentially leading to increased adoption rates.
Growing Demand for Home Healthcare Solutions
The growing demand for home healthcare solutions is significantly influencing the point-of-care-glucose-testing market. As patients increasingly prefer to manage their health from home, the need for convenient and reliable glucose testing devices has surged. This trend is driven by the desire for autonomy in health management and the convenience of at-home testing. The point-of-care-glucose-testing market is responding to this demand by offering user-friendly devices that allow patients to monitor their glucose levels without frequent visits to healthcare facilities. Additionally, the aging population in the US, which often requires ongoing glucose monitoring, is likely to further drive this trend. As home healthcare continues to gain traction, the point-of-care-glucose-testing market is expected to expand, catering to the needs of patients seeking accessible monitoring solutions.
Technological Innovations in Testing Devices
Technological innovations are significantly impacting the point-of-care-glucose-testing market. Advances in biosensor technology, microfluidics, and connectivity features are enhancing the accuracy and usability of glucose testing devices. For instance, the integration of smartphone applications with testing devices allows for real-time data tracking and analysis, which is appealing to tech-savvy consumers. The market is witnessing the introduction of non-invasive glucose monitoring technologies, which could revolutionize patient experiences. As these innovations continue to emerge, they are likely to attract investment and drive competition within the market. The point-of-care-glucose-testing market stands to benefit from these advancements, as they align with the growing demand for user-friendly and efficient healthcare solutions.