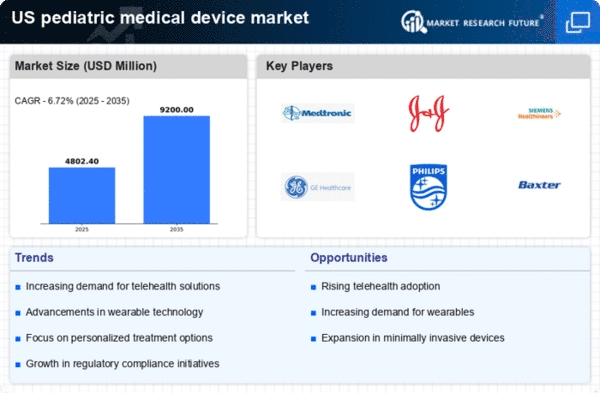
Technological Innovations
Technological advancements play a crucial role in shaping the pediatric medical-device market. Innovations such as miniaturization, enhanced imaging techniques, and telemedicine solutions are transforming how pediatric care is delivered. For instance, the integration of artificial intelligence and machine learning in diagnostic tools is improving accuracy and efficiency in identifying pediatric conditions. The market for pediatric medical devices is projected to grow at a CAGR of around 8% over the next few years, driven by these technological innovations. As manufacturers continue to invest in research and development, the availability of cutting-edge devices tailored for children is expected to increase, further propelling market growth.
Rising Pediatric Population
The pediatric medical-device market is experiencing growth due to the increasing pediatric population in the United States. According to the U.S. Census Bureau, children aged 0-17 years represent approximately 22% of the total population. This demographic trend suggests a growing demand for medical devices tailored to children, as healthcare providers seek to address the unique needs of this age group. The rising number of pediatric patients necessitates the development of specialized devices, which could lead to innovations in design and functionality. Furthermore, as the population of children continues to expand, this market is likely to see a corresponding increase in investment and research aimed at improving health outcomes for younger patients..
Increased Healthcare Expenditure
The pediatric medical-device market is positively influenced by rising healthcare expenditure in the United States. According to the Centers for Medicare & Medicaid Services, national health spending is projected to grow at an average rate of 5.4% annually, reaching nearly $6 trillion by 2027. This increase in healthcare spending is likely to enhance access to advanced medical devices for pediatric patients. As hospitals and healthcare facilities allocate more resources towards pediatric care, the demand for specialized medical devices is expected to rise. This trend indicates a favorable environment for manufacturers in the pediatric medical-device market, as they can anticipate greater investment in innovative solutions that cater to the needs of children.
Regulatory Support for Pediatric Devices
Regulatory support for pediatric medical devices is a significant driver in the market. The U.S. Food and Drug Administration (FDA) has implemented initiatives aimed at facilitating the development and approval of medical devices for children. Programs such as the Pediatric Device Consortia Grant Program encourage innovation by providing funding and resources to support the creation of devices specifically designed for pediatric use. This regulatory environment fosters collaboration between manufacturers and regulatory bodies, potentially leading to a more efficient approval process. As a result, the pediatric medical-device market is likely to see an influx of new products that meet the unique needs of children, enhancing treatment options and improving health outcomes.
Growing Awareness of Pediatric Health Issues
There is a growing awareness of pediatric health issues among parents and healthcare providers, which is driving demand in the pediatric medical-device market. Increased education and advocacy surrounding childhood diseases and conditions have led to a heightened focus on early diagnosis and treatment. This awareness encourages parents to seek medical attention for their children, resulting in a higher utilization of medical devices designed specifically for pediatric patients. As healthcare professionals become more attuned to the unique challenges faced by children, the pediatric medical-device market is likely to benefit from an increase in the development and adoption of specialized devices that address these health concerns.