Rising Demand for Personalized Medicine
The shift towards personalized medicine is significantly influencing the mitochondrial myopathies market. As understanding of genetic factors in mitochondrial disorders improves, there is a growing emphasis on tailoring treatments to individual patient profiles. This trend is likely to enhance the efficacy of therapies and improve patient adherence to treatment regimens. The market for personalized medicine is projected to expand, with estimates indicating a growth rate of approximately 10% annually over the next few years. By focusing on individualized approaches, healthcare providers can optimize treatment outcomes and reduce the burden of side effects. This paradigm shift not only benefits patients but also encourages pharmaceutical companies to invest in the development of targeted therapies, thereby driving the mitochondrial myopathies market forward.
Increased Collaboration Among Stakeholders
Collaboration among various stakeholders, including pharmaceutical companies, research institutions, and patient advocacy groups, is emerging as a key driver in the mitochondrial myopathies market. These partnerships facilitate knowledge sharing, resource pooling, and the acceleration of clinical trials for new therapies. By working together, stakeholders can address the complexities of mitochondrial disorders more effectively, leading to faster development and approval of innovative treatments. The establishment of collaborative networks is likely to enhance the visibility of mitochondrial diseases, fostering greater awareness and understanding among healthcare providers. This increased collaboration may also attract additional funding and support for research initiatives, further propelling the mitochondrial myopathies market. As stakeholders unite to tackle these challenges, the potential for advancements in treatment options becomes more promising.
Rising Prevalence of Mitochondrial Disorders
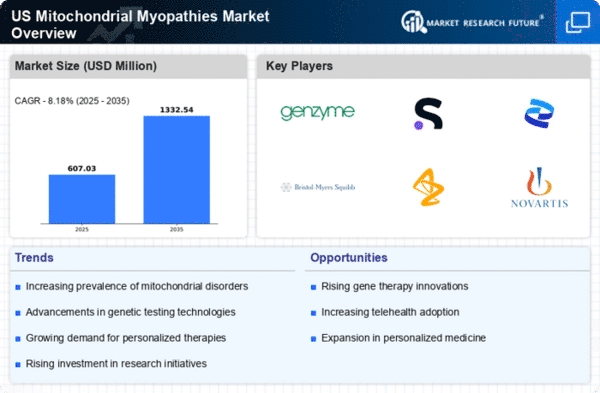
The increasing incidence of mitochondrial disorders in the US is a crucial driver for the mitochondrial myopathies market. Recent estimates suggest that mitochondrial diseases affect approximately 1 in 4,000 individuals, leading to a growing patient population requiring specialized care. This rise in prevalence is likely to stimulate demand for diagnostic tools and therapeutic interventions, thereby expanding the market. As healthcare providers become more aware of these conditions, the need for effective treatments and management strategies will intensify. Consequently, pharmaceutical companies are focusing on developing targeted therapies, which could potentially enhance patient outcomes and drive market growth. The increasing burden of these disorders on healthcare systems may also prompt government and private sector investments in research and development, further propelling the mitochondrial myopathies market forward.
Growing Investment in Research and Development
The mitochondrial myopathies market is witnessing a surge in investment directed towards research and development (R&D). Pharmaceutical companies and academic institutions are increasingly allocating resources to explore novel therapeutic approaches for mitochondrial disorders. This trend is driven by the recognition of unmet medical needs and the potential for innovative treatments to address these complex conditions. In recent years, funding for mitochondrial research has seen a notable increase, with estimates suggesting that R&D expenditures in this area could reach $500 million annually by 2026. Such investments are expected to yield breakthroughs in drug development, enhancing the therapeutic landscape for patients. As new therapies emerge from the pipeline, the mitochondrial myopathies market is poised for substantial growth, reflecting the commitment to improving patient outcomes.
Technological Advancements in Diagnostic Tools
Innovations in diagnostic technologies are significantly impacting the mitochondrial myopathies market. The development of advanced genetic testing methods, such as next-generation sequencing, has improved the accuracy and speed of diagnosing mitochondrial disorders. These advancements enable healthcare professionals to identify conditions earlier, which is crucial for effective treatment planning. The market for diagnostic tools is projected to grow, with estimates indicating a compound annual growth rate (CAGR) of around 8% over the next five years. Enhanced diagnostic capabilities not only facilitate timely interventions but also contribute to better patient management, thereby increasing the overall demand for related therapies. As more patients receive accurate diagnoses, the mitochondrial myopathies market is likely to experience a corresponding rise in therapeutic options and healthcare expenditures.