Rising Cybersecurity Threats
the medical device-security market is increasingly affected by the rise in cybersecurity threats targeting healthcare systems. As medical devices become more interconnected, they present attractive targets for cybercriminals. Reports indicate that nearly 50% of healthcare organizations experienced a data breach in the past year, highlighting the urgent need for enhanced security measures. This trend compels manufacturers to invest in robust security protocols to protect sensitive patient data and ensure device integrity. Consequently, the demand for advanced security solutions within the medical device-security market is expected to grow significantly, as stakeholders prioritize safeguarding their systems against potential attacks.
Regulatory Compliance Requirements
Regulatory compliance is a crucial factor influencing the medical device-security market. The introduction of stringent regulations by agencies such as the FDA mandates that manufacturers implement security measures to protect patient data and device functionality. Compliance with these regulations is not only essential for market entry but also for maintaining consumer trust. As the regulatory landscape evolves, companies are increasingly required to demonstrate their commitment to security, driving demand for innovative solutions within the medical device-security market. This trend suggests that adherence to regulatory standards will continue to shape the market dynamics in the coming years.
Growing Awareness of Patient Privacy
The growing awareness of patient privacy is a pivotal driver for the medical device-security market. As patients become more informed about their rights regarding personal data, healthcare providers are under pressure to ensure the confidentiality and security of sensitive information. This heightened awareness has led to increased scrutiny of medical devices and their security features. Consequently, manufacturers are compelled to enhance their security protocols to meet consumer expectations and regulatory standards. The medical device-security market is likely to benefit from this trend, as organizations strive to build trust with patients by prioritizing data protection.
Technological Advancements in Medical Devices
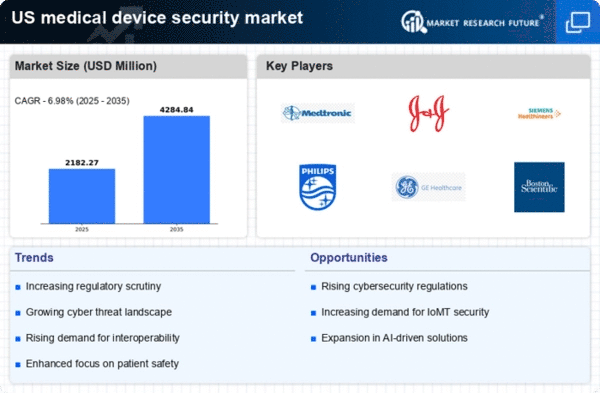
The rapid evolution of technology in medical devices is a key driver for the medical device-security market. Innovations such as IoT-enabled devices and remote monitoring systems enhance patient care but also introduce new vulnerabilities. As these devices become more sophisticated, the potential for exploitation increases, necessitating the implementation of comprehensive security measures. The market is projected to reach $10 billion by 2027, driven by the need for secure devices that comply with stringent regulations. Manufacturers are thus compelled to integrate security features into their products from the design phase, ensuring that the medical device-security market remains a critical focus area.
Increased Investment in Healthcare IT Security
Investment in healthcare IT security is a significant driver for the medical device-security market. With healthcare organizations allocating approximately $125 billion annually to cybersecurity, the emphasis on protecting medical devices has intensified. This financial commitment reflects a growing recognition of the importance of securing medical technology against cyber threats. As healthcare providers seek to comply with regulatory requirements and protect patient data, the demand for specialized security solutions is likely to rise. This trend indicates a robust growth trajectory for the medical device-security market, as organizations prioritize investments in advanced security technologies.