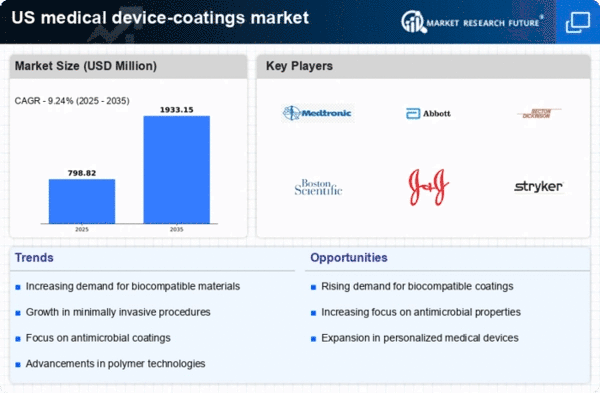
The US Medical Device Coatings Market is characterized by a dynamic landscape of key players striving to innovate and differentiate their offerings in a highly competitive environment. The market is driven by the increasing demand for advanced medical devices with enhanced performance attributes such as biocompatibility, durability, and reduced friction.
Companies are investing heavily in research and development, aiming to develop coatings that not only meet regulatory standards but also improve patient outcomes. The competitive insights reveal that strategic alliances, mergers, and acquisitions play a crucial role in shaping market dynamics, enabling companies to leverage synergies and expand their product portfolios.
Additionally, the regulatory landscape and the emphasis on quality assurance significantly influence competitive strategies, as companies must navigate complex compliance requirements while striving for market leadership.
Smith and Nephew
Smith and Nephew has established a strong presence in the US Medical Device Coatings Market through its commitment to innovation and quality. The company's focus on developing advanced coatings for surgical products and wound management devices allows it to cater to various healthcare needs effectively.
Smith and Nephew's strengths lie in its robust research and development capabilities, which enable it to introduce state-of-the-art coatings that enhance the efficacy of its devices. Moreover, the company’s continuous efforts to forge partnerships and collaborate with healthcare practitioners have further solidified its position in the market.
By prioritizing customer feedback and clinical efficacy, Smith and Nephew successfully differentiates its offerings, thereby enhancing its competitive edge amidst a growing array of alternatives.
Boston Scientific
Boston Scientific holds a significant position in the US Medical Device Coatings Market, renowned for its innovative products and comprehensive service offerings. The company specializes in developing coatings for various medical devices, including cardiovascular, endoscopy, and urology products.
Its extensive portfolio reflects Boston Scientific's commitment to addressing diverse clinical needs through advanced technology. The company's strengths are further accentuated by its strategic approach to mergers and acquisitions, which has allowed it to enhance its capabilities and expand its market reach.
With a focus on quality and performance, Boston Scientific consistently aims to deliver cutting-edge solutions that meet regulatory standards while improving patient outcomes. The company’s dedication to R&D positions it favorably in the competitive landscape, enabling it to respond effectively to emerging trends and technologies in the medical device coatings sector.





















Leave a Comment