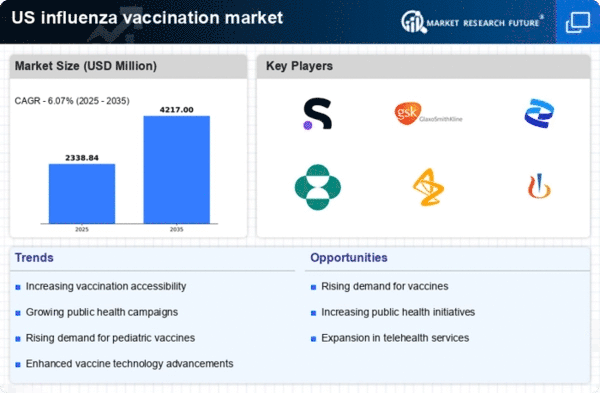
The influenza vaccination market is characterized by a competitive landscape that is increasingly shaped by innovation, strategic partnerships, and a focus on supply chain resilience. Key players such as Pfizer (US), Merck & Co. (US), and GlaxoSmithKline (GB) are actively pursuing strategies that emphasize research and development, regional expansion, and digital transformation. These companies are not only enhancing their product offerings but also adapting to changing consumer demands and regulatory environments, which collectively influences the market dynamics and competitive positioning. In terms of business tactics, companies are localizing manufacturing to reduce lead times and optimize supply chains, which is particularly crucial in the context of seasonal demand fluctuations. The market appears moderately fragmented, with several players vying for market share, yet the collective influence of major companies like Sanofi (FR) and AstraZeneca (GB) is significant. Their operational strategies, including mergers and acquisitions, are likely to consolidate market power and enhance competitive advantages. In October 2025, Pfizer (US) announced a strategic partnership with a leading technology firm to integrate AI-driven analytics into its vaccine development processes. This move is expected to streamline research timelines and improve the efficacy of vaccine formulations, thereby positioning Pfizer as a frontrunner in innovation within the market. The integration of advanced technologies could potentially redefine how vaccines are developed and distributed, enhancing overall public health outcomes. In September 2025, Merck & Co. (US) launched a new initiative aimed at increasing vaccination rates among underserved populations. This initiative includes mobile vaccination units and community outreach programs, reflecting a commitment to social responsibility and public health. By addressing disparities in vaccine access, Merck is not only enhancing its corporate image but also expanding its market reach, which may lead to increased sales and brand loyalty. In August 2025, GlaxoSmithKline (GB) expanded its manufacturing capabilities in the US, investing approximately $200 million in a new facility dedicated to influenza vaccine production. This expansion is indicative of GSK's long-term commitment to meeting growing demand and ensuring supply chain reliability. Such investments are likely to enhance GSK's competitive positioning and operational efficiency, allowing for quicker responses to market needs. As of November 2025, current trends in the influenza vaccination market include a strong emphasis on digitalization, sustainability, and the integration of AI technologies. Strategic alliances are increasingly shaping the competitive landscape, enabling companies to leverage shared resources and expertise. Looking ahead, it appears that competitive differentiation will evolve from traditional price-based competition to a focus on innovation, technological advancements, and robust supply chain management. This shift may ultimately lead to improved vaccine accessibility and effectiveness, benefiting public health on a broader scale.