Rising Healthcare Expenditure
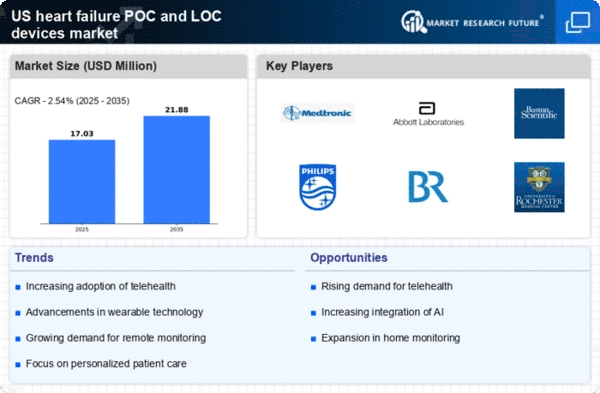
The increasing healthcare expenditure in the US is a significant driver for the heart failure-poc-and-loc-devices market. As healthcare spending continues to rise, estimated to reach $6 trillion by 2027, there is a growing emphasis on investing in advanced medical technologies. This trend is particularly relevant for heart failure management, where the costs associated with hospitalizations and treatments are substantial. The heart failure-poc-and-loc-devices market stands to benefit from this increased investment, as healthcare providers seek cost-effective solutions that improve patient outcomes and reduce overall healthcare costs. Furthermore, the focus on value-based care is likely to drive the adoption of innovative devices that demonstrate clear clinical benefits and cost savings.
Increased Focus on Home Healthcare
The shift towards home healthcare is a notable trend impacting the heart failure-poc-and-loc-devices market. With an aging population and a growing preference for at-home treatment options, healthcare providers are increasingly adopting POC and LOC devices that allow for remote monitoring and management of heart failure. This trend is supported by the desire to reduce hospital readmissions and improve patient quality of life. According to recent studies, home healthcare can lead to a reduction in hospital visits by up to 25%. As a result, the heart failure-poc-and-loc-devices market is likely to see a surge in demand for devices that facilitate home monitoring, enabling patients to manage their conditions more effectively while remaining in the comfort of their homes.
Rising Prevalence of Heart Failure
The increasing incidence of heart failure in the US is a primary driver for the heart failure-poc-and-loc-devices market. According to the American Heart Association, approximately 6.2 million adults in the US are living with heart failure, a figure that is projected to rise as the population ages. This growing patient base necessitates the development and adoption of point-of-care (POC) and location-based (LOC) devices to facilitate timely diagnosis and management. The heart failure POC and LOC devices market is expected to expand as healthcare providers seek innovative solutions for effective patient monitoring and improved outcomes. Furthermore, the economic burden associated with heart failure, estimated at $30 billion annually in the US, underscores the urgent need for efficient management tools, thereby driving demand for advanced devices.
Technological Innovations in Device Design
Innovations in technology are significantly influencing the heart failure-poc-and-loc-devices market. The advent of miniaturized sensors, wireless connectivity, and advanced data analytics has led to the development of sophisticated devices that can monitor heart failure symptoms in real-time. These innovations not only enhance patient engagement but also improve clinical decision-making. For instance, wearable devices that track vital signs and provide alerts to healthcare providers are becoming increasingly popular. The heart failure-poc-and-loc-devices market is likely to benefit from these advancements, as they enable more personalized and proactive care. As technology continues to evolve, the integration of artificial intelligence and machine learning into these devices may further enhance their capabilities, potentially transforming patient management strategies.
Growing Awareness and Education on Heart Failure
The rising awareness and education surrounding heart failure are crucial factors influencing the heart failure-poc-and-loc-devices market. Public health campaigns and educational initiatives have significantly improved understanding of heart failure symptoms, risk factors, and management strategies. This heightened awareness encourages patients to seek timely medical attention and adopt preventive measures, thereby increasing the demand for monitoring devices. As patients become more informed about their conditions, they are more likely to utilize POC and LOC devices to manage their heart failure effectively. The heart failure-poc-and-loc-devices market is expected to grow as healthcare systems prioritize patient education and support, ultimately leading to better health outcomes and reduced healthcare costs.