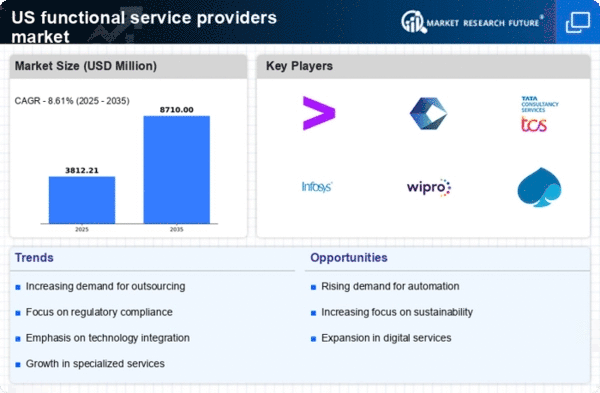
Growing Regulatory Pressures
the functional service-providers-fsp market is greatly affected by the increasing regulatory pressures across various industries. Organizations are compelled to comply with stringent regulations, particularly in sectors such as finance and healthcare. This has led to a heightened demand for service providers that possess a deep understanding of regulatory requirements and can ensure compliance. Recent statistics suggest that companies that engage specialized service providers for compliance-related functions reduce their risk of regulatory penalties by approximately 40%. As regulatory landscapes continue to evolve, the need for knowledgeable functional service providers is expected to expand.
Rising Need for Cost Efficiency
the functional service-providers-fsp market is undergoing a notable shift towards cost efficiency as organizations seek to optimize their operational expenditures. Companies are increasingly outsourcing non-core functions to specialized service providers, which can lead to a reduction in overhead costs. According to recent data, businesses that leverage functional service providers can save up to 30% on operational costs. This trend is particularly pronounced in sectors such as healthcare and finance, where regulatory compliance and specialized expertise are paramount. As firms aim to enhance their bottom line, the demand for functional service-providers is likely to grow, driving innovation and competition within the market.
Adoption of Advanced Technologies
The integration of advanced technologies is transforming the functional service-providers-fsp market. Companies are leveraging automation, artificial intelligence, and data analytics to enhance service delivery and improve efficiency. For instance, the use of AI-driven analytics in project management has been shown to reduce project timelines by up to 25%. As organizations increasingly adopt these technologies, the demand for functional service providers that can offer tech-enabled solutions is likely to rise. This trend not only enhances operational efficiency but also allows businesses to focus on strategic initiatives, thereby driving growth in the functional service-providers-fsp market.
Emphasis on Quality and Expertise
In the functional service-providers-fsp market, there is a growing emphasis on quality and expertise as businesses recognize the value of specialized knowledge. Organizations are increasingly seeking providers that can deliver high-quality services tailored to their specific needs. This trend is evident in industries such as pharmaceuticals, where the demand for specialized clinical trial services has surged. Data indicates that companies that prioritize quality in their service providers experience a 20% increase in project success rates. As the market evolves, the focus on expertise is expected to intensify, compelling service providers to enhance their capabilities and offerings.
Shift Towards Flexible Service Models
the functional service-providers-fsp market is experiencing a shift towards more flexible service models as businesses adapt to changing market dynamics. Organizations are increasingly favoring service providers that offer customizable solutions, allowing them to scale services according to their needs. This flexibility is particularly beneficial in industries that experience fluctuating demand, such as retail and logistics. Data indicates that companies utilizing flexible service models can improve their operational agility by up to 35%. As the market continues to evolve, the demand for adaptable service offerings is likely to grow, shaping the future landscape of the functional service-providers-fsp market.