Regulatory Approvals and Market Access
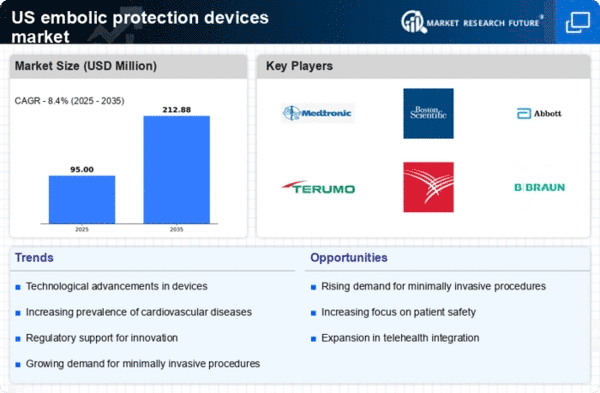
The regulatory landscape for medical devices in the US plays a crucial role in shaping the embolic protection-devices market. Recent efforts by regulatory bodies to streamline the approval process for innovative medical devices have created a more favorable environment for manufacturers. This regulatory support is likely to encourage the introduction of new and improved embolic protection devices, thereby expanding market access. As companies navigate the regulatory framework more efficiently, the availability of advanced devices in the market is expected to increase, ultimately benefiting healthcare providers and patients alike. The positive regulatory climate is anticipated to drive growth in the embolic protection-devices market.
Technological Innovations in Device Design
Innovations in the design and functionality of embolic protection devices are significantly influencing the market landscape. Recent advancements have led to the development of devices that are more efficient, user-friendly, and capable of providing better protection during surgical procedures. For instance, the introduction of next-generation filters and balloons has improved the efficacy of these devices, leading to enhanced patient safety. The embolic protection-devices market is projected to grow as healthcare facilities increasingly adopt these advanced technologies, which not only improve procedural outcomes but also reduce the overall cost of care. The integration of digital technologies, such as real-time imaging and monitoring, further enhances the appeal of these devices.
Rising Incidence of Cardiovascular Diseases
The increasing prevalence of cardiovascular diseases in the US is a primary driver for the embolic protection-devices market. As per recent statistics, cardiovascular diseases account for approximately 697,000 deaths annually, representing about 1 in every 5 deaths. This alarming trend necessitates advanced medical interventions, including embolic protection devices, to mitigate risks during procedures such as percutaneous coronary interventions. The demand for these devices is expected to rise as healthcare providers seek to enhance patient outcomes and reduce complications associated with embolic events. Consequently, the embolic protection-devices market is likely to experience substantial growth, driven by the urgent need for effective solutions in managing cardiovascular health.
Increasing Investment in Healthcare Infrastructure
The US healthcare sector is witnessing a surge in investment aimed at enhancing medical infrastructure, which is positively impacting the embolic protection-devices market. With the government and private entities allocating substantial funds to upgrade healthcare facilities, there is a corresponding increase in the procurement of advanced medical devices, including embolic protection devices. This trend is likely to continue as hospitals and clinics strive to provide state-of-the-art care to patients. The infusion of capital into healthcare infrastructure not only facilitates the acquisition of new technologies but also promotes research and development in the embolic protection-devices market, fostering innovation and growth.
Growing Awareness and Education Among Healthcare Professionals
There is a notable increase in awareness and education regarding the importance of embolic protection devices among healthcare professionals in the US. As medical practitioners become more informed about the benefits of these devices, their adoption rates are likely to rise. Educational initiatives and training programs are being implemented to ensure that healthcare providers are well-versed in the use of embolic protection devices during high-risk procedures. This growing knowledge base is expected to drive demand within the embolic protection-devices market, as practitioners recognize the potential to improve patient outcomes and reduce complications associated with embolic events.