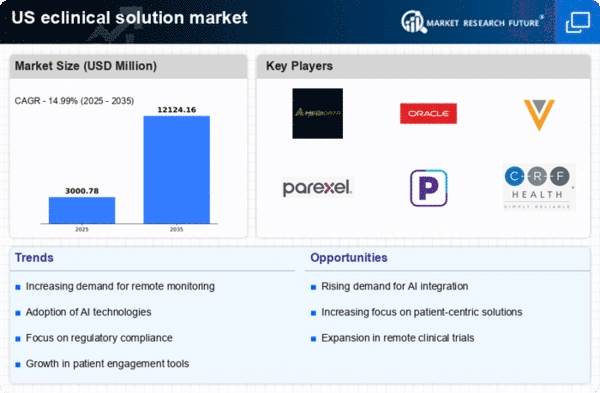
Rising Demand for Clinical Trials
The eclinical solution market experiences a notable surge in demand for clinical trials, driven by the increasing need for innovative therapies and drugs. As pharmaceutical companies and research organizations strive to expedite the drug development process, the utilization of eclinical solutions becomes essential. In 2025, the market for clinical trials in the US is projected to reach approximately $20 billion, indicating a robust growth trajectory. This demand is further fueled by the need for efficient data management and regulatory compliance, which eclinical solutions adeptly provide. The integration of advanced technologies, such as artificial intelligence and machine learning, into eclinical solutions enhances the efficiency of clinical trials, thereby attracting more stakeholders to invest in these technologies. Consequently, the rising demand for clinical trials significantly propels the growth of the eclinical solution market.
Emphasis on Patient-Centric Approaches
The eclinical solution market is increasingly influenced by the emphasis on patient-centric approaches in clinical research. Organizations are recognizing the importance of patient engagement and experience in the success of clinical trials. In 2025, it is estimated that around 70% of clinical trials will incorporate patient-centric methodologies, driving the demand for eclinical solutions that facilitate this shift. These solutions enable better communication with participants, enhance data collection through mobile applications, and provide real-time feedback mechanisms. By prioritizing patient needs and preferences, organizations can improve recruitment and retention rates in clinical trials. This growing emphasis on patient-centric approaches not only enhances the quality of clinical research but also propels the growth of the eclinical solution market as stakeholders seek innovative solutions to meet these evolving demands.
Growing Focus on Regulatory Compliance
Regulatory compliance remains a critical driver for the eclinical solution market, as organizations strive to adhere to stringent guidelines set forth by regulatory bodies. In the US, the Food and Drug Administration (FDA) continues to emphasize the importance of data integrity and patient safety in clinical trials. As a result, eclinical solutions that facilitate compliance with these regulations are increasingly sought after. The market is projected to grow by approximately 12% in 2025, reflecting the heightened focus on regulatory adherence. Eclinical solutions that offer features such as audit trails, electronic signatures, and real-time monitoring are particularly valuable in ensuring compliance. This growing emphasis on regulatory compliance not only enhances the credibility of clinical trials but also fosters trust among stakeholders, thereby propelling the eclinical solution market forward.
Technological Advancements in Data Management
Technological advancements play a pivotal role in shaping the eclinical solution market, particularly in the realm of data management. The increasing complexity of clinical data necessitates sophisticated solutions that can handle vast amounts of information efficiently. In 2025, the eclinical solution market is expected to witness a growth rate of around 15%, largely attributed to innovations in data analytics and cloud computing. These advancements enable real-time data access and analysis, facilitating better decision-making processes for clinical researchers. Moreover, the integration of electronic data capture (EDC) systems within eclinical solutions streamlines data collection and enhances data integrity. As organizations prioritize data-driven approaches, the demand for advanced eclinical solutions that leverage these technological advancements is likely to rise, further driving market growth.
Increased Investment in Research and Development
Investment in research and development (R&D) is a significant driver of the eclinical solution market, as organizations allocate substantial resources to discover new treatments and therapies. In 2025, R&D spending in the pharmaceutical sector is anticipated to exceed $200 billion in the US, creating a favorable environment for the adoption of eclinical solutions. These solutions streamline the R&D process by providing tools for efficient data collection, analysis, and reporting. Furthermore, the competitive landscape compels organizations to leverage eclinical solutions to enhance their operational efficiency and reduce time-to-market for new products. As R&D investments continue to rise, the demand for eclinical solutions that support these initiatives is likely to grow, thereby contributing to the overall expansion of the market.