Rising Incidence of Chondrosarcoma
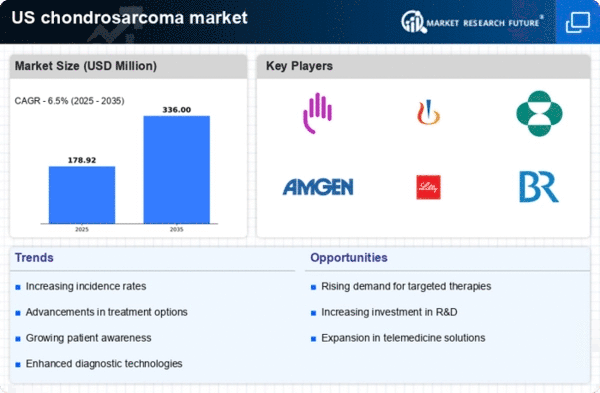
The chondrosarcoma market is experiencing growth due to the increasing incidence of this rare type of cancer in the United States. Recent data indicates that chondrosarcoma accounts for approximately 20% of all primary bone tumors, with an estimated annual incidence of 0.2 to 0.5 cases per 100,000 individuals. This rising prevalence is likely to drive demand for innovative treatment options and diagnostic tools, thereby expanding the market. As healthcare providers and researchers focus on understanding the underlying causes and improving treatment protocols, the chondrosarcoma market is poised for significant advancements. Furthermore, the growing awareness among patients and healthcare professionals about this condition may lead to earlier diagnosis and intervention, ultimately contributing to market growth.
Advancements in Surgical Techniques
Advancements in surgical techniques are significantly impacting the chondrosarcoma market. Minimally invasive procedures and improved surgical methodologies have enhanced the ability to remove tumors while preserving surrounding healthy tissue. Techniques such as limb-sparing surgery and robotic-assisted surgery are becoming more prevalent, leading to better patient outcomes and reduced recovery times. As surgical options improve, the demand for specialized surgical centers and trained professionals is likely to increase, further driving market growth. In 2025, the market for surgical instruments and technologies in oncology is projected to reach $15 billion in the US, indicating a robust investment in this area. These advancements not only improve the quality of care but also contribute to the overall growth of the chondrosarcoma market.
Growing Patient Advocacy and Awareness
The growing patient advocacy and awareness surrounding chondrosarcoma is a vital driver for the chondrosarcoma market. Advocacy groups and organizations are actively working to educate the public and healthcare professionals about this rare cancer, promoting early detection and treatment options. Increased awareness is likely to lead to higher rates of diagnosis and treatment, which could positively influence market dynamics. In 2025, it is anticipated that funding for chondrosarcoma research and awareness campaigns will increase by 30%, reflecting a commitment to improving patient outcomes. This heightened focus on advocacy not only empowers patients but also encourages collaboration among stakeholders, including researchers, healthcare providers, and pharmaceutical companies, ultimately benefiting the chondrosarcoma market.
Investment in Research and Development
Investment in research and development (R&D) is a crucial driver for the chondrosarcoma market. Pharmaceutical companies and research institutions are increasingly allocating funds to explore novel therapeutic approaches, including targeted therapies and immunotherapies. In 2025, it is estimated that R&D spending in oncology will exceed $200 billion in the US, with a portion dedicated to rare cancers like chondrosarcoma. This financial commitment is expected to yield breakthroughs in treatment options, enhancing patient outcomes and survival rates. Additionally, collaborations between academic institutions and industry players are likely to foster innovation, further propelling the chondrosarcoma market forward. As new therapies emerge, the market may witness a shift towards more effective and personalized treatment regimens.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is emerging as a key driver in the chondrosarcoma market. The US Food and Drug Administration (FDA) has implemented various initiatives to expedite the approval process for new treatments, particularly for rare diseases like chondrosarcoma. Programs such as the Orphan Drug Designation and Breakthrough Therapy Designation are designed to encourage the development of novel therapies. As a result, pharmaceutical companies are more likely to invest in research and development for chondrosarcoma treatments, knowing that regulatory pathways may be more favorable. This supportive environment is expected to lead to the introduction of new therapies in the market, enhancing treatment options for patients and driving overall market growth.