Enhanced Patient Support Programs
The cervical dystonia market is benefiting from enhanced patient support programs that aim to improve treatment adherence and patient outcomes. These programs often include educational resources, counseling, and access to healthcare professionals who specialize in managing cervical dystonia. By providing comprehensive support, these initiatives help patients navigate their treatment options and manage their condition more effectively. As a result, patient satisfaction and treatment adherence are likely to improve, which could lead to better health outcomes. The expansion of such support programs is expected to positively influence the cervical dystonia market by fostering a more informed patient population that actively engages in their treatment.
Regulatory Support for New Treatments
Regulatory support for new treatments is a critical factor influencing the cervical dystonia market. The US Food and Drug Administration (FDA) has been actively facilitating the approval process for innovative therapies aimed at treating cervical dystonia. This supportive regulatory environment encourages pharmaceutical companies to invest in the development of new medications and treatment modalities. As a result, the market is witnessing an influx of novel therapies that could potentially transform patient care. The expedited approval pathways and incentives for orphan drugs are likely to enhance the availability of effective treatments, thereby driving growth in the cervical dystonia market.
Technological Innovations in Treatment
Technological advancements are playing a crucial role in shaping the cervical dystonia market. Innovations such as botulinum toxin injections, deep brain stimulation, and new oral medications are enhancing treatment efficacy and patient outcomes. For instance, the introduction of new formulations of botulinum toxin has shown improved results in reducing muscle spasms associated with cervical dystonia. Furthermore, the market for these treatments is projected to reach approximately $1.5 billion by 2027, indicating a robust growth trajectory. These technological innovations not only improve the quality of life for patients but also stimulate market growth by attracting investment and encouraging research and development in the cervical dystonia market.
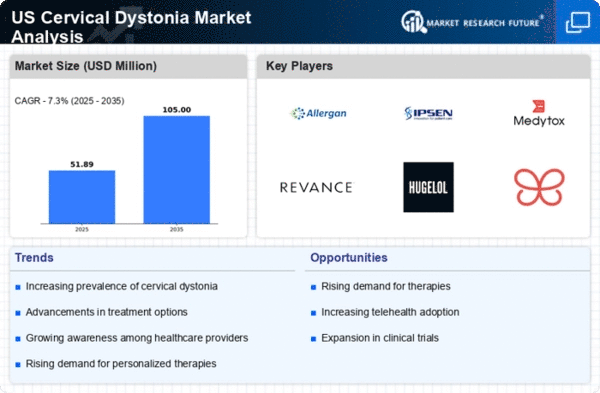
Increasing Prevalence of Cervical Dystonia
The cervical dystonia market is experiencing growth due to the rising prevalence of this neurological disorder in the US. Recent estimates suggest that cervical dystonia affects approximately 3.2 per 100,000 individuals annually. This increasing incidence is likely to drive demand for effective treatment options, thereby expanding the market. As awareness of the condition grows, more patients are seeking medical advice, leading to higher diagnosis rates. Consequently, healthcare providers are focusing on developing innovative therapies to address the needs of this patient population. The increasing prevalence of cervical dystonia is expected to significantly impact the cervical dystonia market, as it creates a larger patient base requiring ongoing management and treatment.
Growing Investment in Research and Development
Investment in research and development (R&D) is a significant driver of the cervical dystonia market. Pharmaceutical companies and research institutions are increasingly allocating resources to explore new therapeutic options and improve existing treatments. This trend is evidenced by the rise in clinical trials focused on cervical dystonia, with over 50 ongoing studies in the US as of late 2025. Such investments are likely to lead to the discovery of novel therapies, which could enhance treatment efficacy and patient adherence. The commitment to R&D not only fosters innovation but also positions the cervical dystonia market for sustained growth as new therapies emerge to meet the needs of patients.