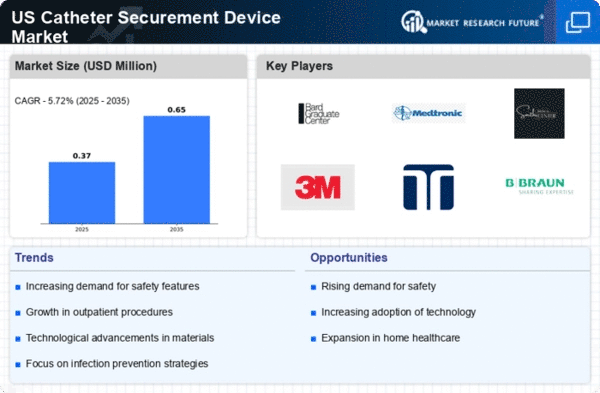
Rising Demand for Enhanced Patient Safety
The catheter securement-device market is experiencing a notable increase in demand driven by the heightened focus on patient safety. Healthcare providers are increasingly prioritizing devices that minimize the risk of catheter-related complications, such as dislodgement and infection. According to recent data, approximately 30% of patients with catheters experience complications, which has prompted hospitals to adopt securement devices that enhance stability and reduce movement. This trend is likely to continue as institutions strive to improve patient outcomes and reduce healthcare costs associated with complications. The catheter securement-device market is thus positioned to grow as more facilities invest in advanced securement solutions that align with their safety protocols.
Emphasis on Cost-Effectiveness in Healthcare
The catheter securement-device market is also being shaped by the increasing emphasis on cost-effectiveness within healthcare systems. As hospitals face pressure to reduce operational costs while maintaining high standards of care, the adoption of cost-efficient securement devices becomes paramount. Studies indicate that effective securement can lead to a reduction in catheter-related complications, which in turn lowers overall treatment costs. This financial incentive encourages healthcare providers to invest in securement devices that not only ensure patient safety but also contribute to long-term savings. Thus, the catheter securement-device market is likely to see growth as providers seek solutions that align with their budgetary constraints.
Growth in Outpatient and Home Healthcare Services
The expansion of outpatient and home healthcare services is significantly influencing the catheter securement-device market. As more patients receive care outside traditional hospital settings, the need for reliable and effective securement devices has surged. Data indicates that the outpatient care market is projected to grow at a CAGR of 10% over the next five years. This shift necessitates securement devices that are not only effective but also user-friendly for patients and caregivers. Consequently, manufacturers are innovating to create products that cater to this growing segment, ensuring that the catheter securement-device market remains responsive to the evolving healthcare landscape.
Increased Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a critical driver for the catheter securement-device market. As hospitals and clinics upgrade their facilities and technology, there is a corresponding demand for advanced securement devices. Recent reports suggest that healthcare spending in the US is expected to reach $4 trillion by 2025, with a significant portion allocated to improving patient care technologies. This influx of capital allows healthcare providers to procure high-quality securement devices that enhance patient safety and operational efficiency. As a result, the catheter securement-device market is likely to benefit from this trend, as facilities seek to implement the latest innovations in securement technology.
Rising Awareness of Catheter-Associated Complications
The growing awareness of catheter-associated complications is a significant driver for the catheter securement-device market. Healthcare professionals are increasingly educated about the risks associated with catheter use, including infections and dislodgement. This awareness has led to a proactive approach in adopting securement devices that mitigate these risks. Data shows that hospitals implementing securement protocols have reported a 25% decrease in catheter-related infections. As awareness continues to rise, healthcare facilities are likely to prioritize the procurement of securement devices, thereby propelling the growth of the catheter securement-device market.