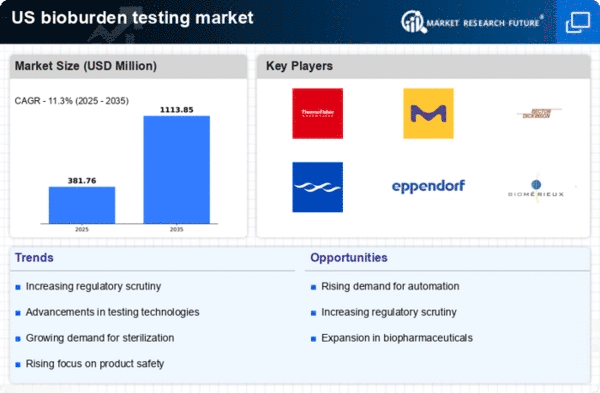
Increased Regulatory Scrutiny
Increased regulatory scrutiny is a significant driver of the bioburden testing market. Regulatory agencies are intensifying their focus on contamination control, leading to more stringent guidelines for manufacturers. This heightened scrutiny compels companies to adopt robust bioburden testing protocols to ensure compliance and avoid potential penalties. The bioburden testing market is likely to benefit from this trend, as organizations invest in testing solutions to meet evolving regulatory demands. The anticipated growth in regulatory requirements could lead to a market expansion of approximately 10% over the next few years, as companies strive to align with these new standards.
Growing Focus on Quality Assurance
Quality assurance has become a pivotal focus for companies operating in the bioburden testing market. With increasing scrutiny from regulatory agencies, organizations are prioritizing the implementation of comprehensive quality management systems. This trend is particularly evident in the pharmaceutical and biotechnology sectors, where the cost of non-compliance can be substantial. As a result, companies are investing in bioburden testing to ensure that their products meet the highest quality standards. The emphasis on quality assurance is expected to contribute to a steady growth rate of around 7% in the bioburden testing market, as firms seek to mitigate risks associated with contamination.
Rising Demand for Sterile Products
The bioburden testing market is experiencing a notable increase in demand for sterile products across various sectors, including pharmaceuticals and medical devices. This surge is largely driven by the need for stringent quality control measures to ensure product safety and efficacy. As regulatory bodies enforce more rigorous standards, manufacturers are compelled to adopt bioburden testing protocols to comply with these requirements. The market for sterile products is projected to grow at a CAGR of approximately 8% over the next few years, further propelling the bioburden testing market. Companies are investing in advanced testing methods to meet these demands, indicating a robust growth trajectory for the industry.
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector is witnessing rapid expansion, which is significantly impacting the bioburden testing market. As more biopharmaceutical companies emerge, the need for effective bioburden testing becomes increasingly critical to ensure product safety and compliance with regulatory standards. The biopharmaceutical market is projected to reach $500 billion by 2026, creating a substantial demand for bioburden testing services. This growth is likely to drive innovation and investment in testing technologies, further enhancing the capabilities of the bioburden testing market. The interplay between biopharmaceutical growth and testing requirements suggests a promising outlook for the industry.
Technological Innovations in Testing Methods
Technological advancements are playing a crucial role in shaping the bioburden testing market. Innovations such as rapid testing methods and automated systems are enhancing the efficiency and accuracy of bioburden testing. These technologies not only reduce the time required for testing but also improve the reliability of results, which is essential for maintaining compliance with industry standards. The introduction of real-time monitoring systems is also gaining traction, allowing for immediate detection of contamination. As these technologies become more accessible, they are likely to drive growth in the bioburden testing market, with an estimated increase in market size by 15% over the next five years.