Increased Government Funding
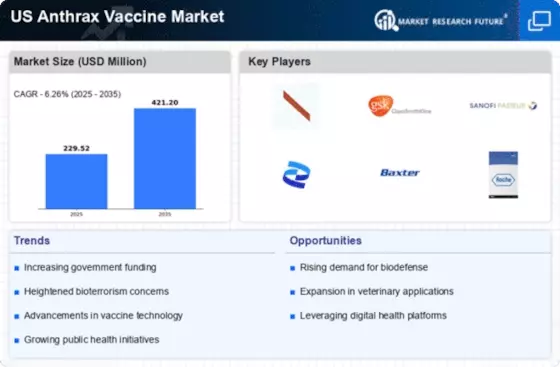
The US Anthrax Vaccine Market is experiencing a surge in government funding aimed at enhancing national security and public health preparedness. The Department of Defense and the Department of Health and Human Services have allocated substantial budgets for the procurement and development of anthrax vaccines. In 2025, the federal budget for biodefense initiatives reached approximately 1.5 billion USD, with a significant portion directed towards anthrax vaccine research and production. This financial commitment underscores the government's recognition of the potential threat posed by anthrax as a bioterrorism agent. Consequently, increased funding is likely to stimulate innovation and production capacity within the US Anthrax Vaccine Market, fostering a more robust response to potential biological threats.
Rising Bioterrorism Threat Perception
The perception of bioterrorism threats has escalated in recent years, significantly impacting the US Anthrax Vaccine Market. High-profile incidents and intelligence reports have heightened awareness of the potential use of anthrax as a biological weapon. This growing concern has prompted both public and private sectors to prioritize the development and stockpiling of anthrax vaccines. According to the Centers for Disease Control and Prevention, the likelihood of anthrax being used in a bioterrorism attack remains a critical issue. As a result, the demand for effective vaccines is expected to rise, driving growth in the US Anthrax Vaccine Market as stakeholders seek to mitigate risks associated with bioterrorism.
Public Health Preparedness Initiatives
Public health preparedness initiatives are increasingly influencing the US Anthrax Vaccine Market. The federal and state governments are implementing comprehensive strategies to enhance readiness for biological threats, including anthrax outbreaks. Programs aimed at improving surveillance, response capabilities, and vaccination campaigns are being prioritized. The National Strategy for Countering Biological Threats emphasizes the importance of maintaining a robust vaccine stockpile and ensuring rapid distribution during emergencies. This proactive approach is expected to drive demand for anthrax vaccines, as public health agencies seek to protect populations from potential outbreaks. Consequently, the US Anthrax Vaccine Market is likely to benefit from these initiatives, fostering collaboration between government entities and vaccine manufacturers.
Growing Awareness of Occupational Hazards
There is a growing awareness of occupational hazards associated with anthrax exposure, particularly among professionals in high-risk fields such as veterinary medicine, agriculture, and laboratory research. This heightened awareness is influencing the US Anthrax Vaccine Market as organizations and employers recognize the importance of vaccination for their employees. Regulatory bodies, including the Occupational Safety and Health Administration, are advocating for vaccination programs to mitigate risks in these sectors. As more companies implement mandatory vaccination policies, the demand for anthrax vaccines is expected to increase. This trend not only enhances workplace safety but also contributes to the overall growth of the US Anthrax Vaccine Market.
Technological Innovations in Vaccine Development
Technological advancements in vaccine development are playing a pivotal role in shaping the US Anthrax Vaccine Market. Innovations such as recombinant DNA technology and novel adjuvants are enhancing the efficacy and safety profiles of anthrax vaccines. For instance, the recent introduction of next-generation vaccines has shown promise in eliciting stronger immune responses with fewer doses. The US Food and Drug Administration has been actively involved in facilitating the approval of these innovative products, which could lead to a more competitive market landscape. As these technologies continue to evolve, they are likely to attract investment and research efforts, further propelling the growth of the US Anthrax Vaccine Market.