Increasing Demand for Targeted Therapies
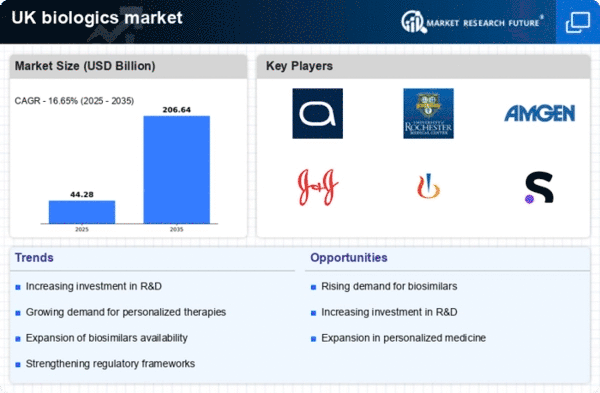
The biologics market in the UK is experiencing a notable surge in demand for targeted therapies. This trend is driven by the growing prevalence of chronic diseases, such as cancer and autoimmune disorders, which require more precise treatment options. According to recent data, the market for targeted biologics is projected to reach approximately £10 billion by 2026, reflecting a compound annual growth rate (CAGR) of around 8%. This increasing demand is prompting pharmaceutical companies to invest heavily in research and development, thereby expanding their portfolios in the biologics market. Furthermore, advancements in genomics and proteomics are enabling the identification of specific biomarkers, which enhances the efficacy of targeted therapies. As a result, the biologics market is likely to witness a shift towards more personalized treatment approaches, catering to the unique needs of patients.
Growing Focus on Patient-Centric Approaches
The biologics market in the UK is increasingly shifting towards patient-centric approaches, which are reshaping the landscape of drug development and delivery. Pharmaceutical companies are recognizing the importance of incorporating patient feedback into the design of clinical trials and treatment protocols. This trend is likely to enhance patient adherence and satisfaction, ultimately leading to better health outcomes. Furthermore, the integration of digital health technologies, such as telemedicine and mobile health applications, is facilitating more personalized care and monitoring. As patients become more engaged in their treatment journeys, the demand for biologics that align with their preferences and needs is expected to rise. This patient-centric focus may drive innovation in the biologics market, as companies strive to develop therapies that not only address clinical efficacy but also enhance the overall patient experience.
Regulatory Support for Biologics Development
Regulatory frameworks in the UK are evolving to support the growth of the biologics market. The Medicines and Healthcare products Regulatory Agency (MHRA) is actively streamlining the approval process for biologics, which is likely to expedite the time-to-market for new therapies. Recent initiatives, such as the introduction of accelerated pathways for innovative treatments, are designed to facilitate quicker access to life-saving biologics for patients. This regulatory support is expected to encourage investment in research and development, as companies gain confidence in the approval process. Additionally, the emphasis on post-market surveillance and real-world evidence is fostering a more robust understanding of the long-term safety and efficacy of biologics. As a result, the biologics market is poised for sustained growth, driven by a conducive regulatory environment that promotes innovation and patient access.
Rising Investment in Biopharmaceutical Research
Investment in biopharmaceutical research is a significant driver of growth in the biologics market in the UK. The government and private sector are increasingly allocating funds towards the development of novel biologics, with total investment reaching approximately £3 billion in 2025. This influx of capital is facilitating the exploration of new therapeutic areas and the advancement of existing biologics. Moreover, collaborations between academic institutions and industry players are fostering innovation and accelerating the translation of research into viable products. The UK is also home to several leading research institutions, which enhances its attractiveness as a hub for biopharmaceutical development. As a result, the biologics market is likely to benefit from a robust pipeline of new therapies, ultimately improving patient outcomes and expanding treatment options.
Technological Advancements in Biologics Manufacturing
Technological innovations are playing a crucial role in shaping the biologics market in the UK. The introduction of advanced manufacturing techniques, such as continuous bioprocessing and single-use technologies, is enhancing production efficiency and reducing costs. These advancements are expected to lower the average production cost of biologics by approximately 15% over the next few years. Additionally, automation and digitalization in manufacturing processes are improving quality control and compliance with regulatory standards. As a result, companies are better positioned to meet the increasing demand for biologics while maintaining high-quality standards. The integration of artificial intelligence and machine learning in the development and production phases is also streamlining operations, thereby contributing to the overall growth of the biologics market. This technological evolution is likely to attract further investments and foster innovation within the sector.