Collaboration Between Stakeholders
Collaboration among various stakeholders, including academic institutions, pharmaceutical companies, and patient advocacy groups, is becoming increasingly prevalent in the Tyrosine Hydroxylase Deficiency Market. These partnerships are fostering a multidisciplinary approach to research and treatment development. By pooling resources and expertise, stakeholders can accelerate the discovery of new therapies and improve patient access to existing treatments. Collaborative initiatives are also enhancing data sharing and clinical trial participation, which are critical for advancing the understanding of Tyrosine Hydroxylase Deficiency Market. This trend is likely to create a more robust ecosystem for innovation, ultimately benefiting patients and driving growth in the Tyrosine Hydroxylase Deficiency Market.
Regulatory Support for Orphan Drugs
Regulatory bodies are providing enhanced support for the development of orphan drugs, which are essential for treating rare conditions like Tyrosine Hydroxylase Deficiency Market. Initiatives such as expedited review processes and market exclusivity incentives are encouraging pharmaceutical companies to invest in this niche market. The Tyrosine Hydroxylase Deficiency Market stands to benefit significantly from these regulatory frameworks, as they lower the barriers to entry for new treatments. This supportive environment is likely to lead to an increase in the number of therapies available for patients, thereby expanding the market. As regulatory agencies continue to prioritize rare diseases, the Tyrosine Hydroxylase Deficiency Market is expected to flourish.
Increasing Awareness of Rare Diseases
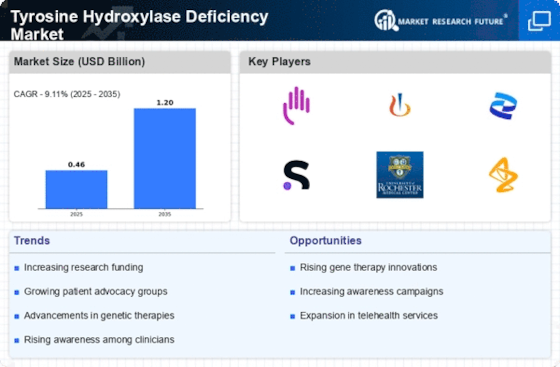
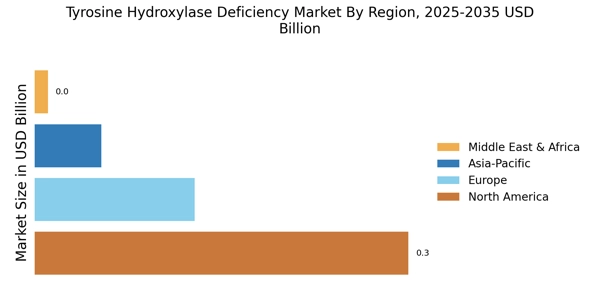
The Tyrosine Hydroxylase Deficiency Market is experiencing a notable increase in awareness regarding rare diseases. This heightened awareness is largely driven by advocacy groups and healthcare professionals who are actively promoting education about Tyrosine Hydroxylase Deficiency Market. As a result, more patients are being diagnosed, which is likely to lead to an increase in demand for treatment options. The prevalence of Tyrosine Hydroxylase Deficiency Market is estimated to be around 1 in 100,000 births, indicating a need for specialized care and therapies. This growing recognition is expected to stimulate research and development efforts, thereby expanding the market for therapeutic interventions in the Tyrosine Hydroxylase Deficiency Market.
Technological Advancements in Treatment
Technological advancements are playing a crucial role in shaping the Tyrosine Hydroxylase Deficiency Market. Innovations in drug formulation and delivery systems are enhancing the efficacy of existing treatments. For instance, the development of enzyme replacement therapies and gene therapies is showing promise in addressing the underlying causes of Tyrosine Hydroxylase Deficiency Market. The market is projected to grow as these advanced therapies become more accessible to patients. Furthermore, the integration of digital health technologies, such as telemedicine and mobile health applications, is facilitating better patient management and follow-up care. This trend is likely to enhance patient outcomes and increase the overall market size in the Tyrosine Hydroxylase Deficiency Market.
Growing Investment in Rare Disease Research
Investment in research and development for rare diseases, including Tyrosine Hydroxylase Deficiency Market, is on the rise. Pharmaceutical companies and research institutions are increasingly allocating resources to explore novel therapeutic approaches. This trend is supported by government incentives and funding programs aimed at fostering innovation in the Tyrosine Hydroxylase Deficiency Market. The global market for rare disease treatments is expected to reach several billion dollars in the coming years, reflecting the lucrative opportunities that exist for stakeholders. As more funding becomes available, it is anticipated that breakthroughs in treatment options will emerge, further propelling the growth of the Tyrosine Hydroxylase Deficiency Market.