Market Trends
Key Emerging Trends in the Thymus Cancer Market
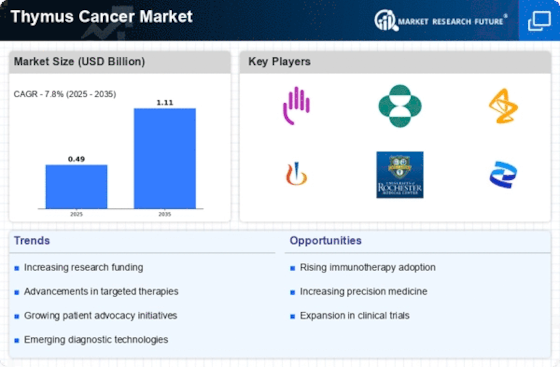
The market for thymus cancer treatment is characterized by its rarity, but it is gaining attention as an emerging concern in the field of oncology. Thymus cancer, while relatively uncommon, poses unique challenges due to its location and varied histological subtypes, necessitating specialized treatment approaches. The market in front of us is quite active and reveals the divergent development of diagnostics for the cancer of thymus. Developed imaging technologies like PET-CT scans and the molecular diagnostics facilitate the discovery of malignancies at an early stage, providing the chance of prompt therapy with the personalise treatment strategy.
Thymus cancer treatment is not without hope. Precision medicine has indeed become a core pillar for devising treatment schemes. Molecular screening and genetic testing empower physicians to adapt strategies according to the unique features displayed by the cancer of their patients thus improving the literal sense and reciprocity of patient care. Pharmacological industries are exploring the molecular mechanisms of thymus cancer in order to design the next generation of targeted therapy. Designated drugs are being developed to be able to work at the molecular level of thymus cancer and this is being done by targeting cancer associated pathways which offers more selective and effective treatments. Given that thymus cancer represents a complex form of cancer, multimodal therapies are currently gaining more popularity. An approach that combines surgery, chemotherapy, radiation and immunotherapy sequentially was found valuable for the same purpose whereas the new approach involves using the above treatments in a coordinated manner focusing on the diverse nature of thymus cancer. The purposeful interactions that are jointly established by governments around the world have had a major impact on the progress of thymus cancer treatment. Scientists and institutions across borders are engaged collaboratively to share their knowledge, pool their resources, and run clinical trials in order to accelerate the creation of new therapies which lead to a better health of people in general.
The regulatory landscape plays a pivotal role in shaping the thymus cancer treatment market. Regulatory agencies are adapting to the unique challenges posed by rare cancers, providing pathways for accelerated approvals and incentives for the development of innovative therapies for thymus cancer. Conducting clinical trials for thymus cancer poses specific challenges due to its rarity and heterogeneity. However, increased awareness, collaboration, and regulatory support are facilitating the design and execution of clinical trials, paving the way for the evaluation of new treatment modalities.

















Leave a Comment