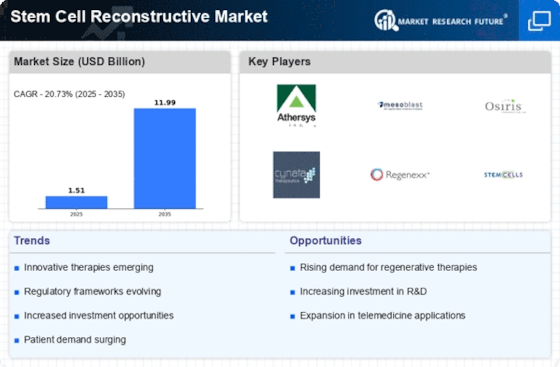
Top Industry Leaders in the Stem Cell Reconstructive Market

Asterias Biotherapeutics Receives IND Approval for Autologous Mesenchymal Stem Cell (MSC) Treatment for Cartilage Reconstruction: This December 2023 development paves the way for clinical trials on this promising therapy for knee cartilage damage.
Osiris Therapeutics Reports Positive Phase 2a Results for Prochymal in Osteoarthritis: December 2023 news further validates the potential of Prochymal, Osiris' mesenchymal stem cell therapy, for treating knee osteoarthritis.
Regen Therapeutics Completes Enrollment for Phase 2 Trial of HRPT-128 for Rotator Cuff Tears: This January 2024 milestone advances the development of Regen's adipose-derived stem cell therapy for this common shoulder injury.
Cyto Therapeutics Partners with Mayo Clinic for Stem Cell Research in Orthopedic Surgery collaboration will focus on developing new stem cell therapies for various musculoskeletal conditions.
NuVasive Invests in Orthox Regenerative Medicine strategic investment in December 2023 strengthens NuVasive's foothold in the regenerative medicine space for spine and orthopedic applications.
Medi-post and Anterogen Form Joint Venture for Development and Commercialization of Stem Cell Therapies partnership combines Medi-post's expertise in cell processing with Anterogen's stem cell platform to accelerate clinical development and market access.
List of Stem Cell Reconstructive Key Companies in the Market
- Cynata (Australia),
- Celyad (Belgium),
- Capricor Therapeutics (Canada),
- Micronit Microfluidics (Netherlands),
- TAKARA BIO INC.(Japan),
- Tigenix (Belgium) Baxter (US),
- Cytori Therapeutics Inc. (US),
- Eleveflow (France),
- Mesoblast Ltd. (Australia),
- NuVasive Inc. (US),
- Osiris Therapeutics, Inc. (US)
- Pfizer Inc. (US)