South Korea Dravet Syndrome Market Overview:
As per MRFR analysis, the South Korea Dravet Syndrome Market Size was estimated at 13.75 (USD Million) in 2023. The South Korea Dravet Syndrome Market Industry is expected to grow from 14.75(USD Million) in 2024 to 36.9 (USD Million) by 2035. The South Korea Dravet Syndrome Market CAGR (growth rate) is expected to be around 8.693% during the forecast period (2025 - 2035).
Key South Korea Dravet Syndrome Market Trends Highlighted
The South Korea Dravet Syndrome Market is seeing major developments as a result of advances in treatment choices and increased awareness among healthcare professionals and families. The South Korean government recognizes the need for improved healthcare solutions for rare diseases, and there is a strong effort to incorporate novel medicines into the healthcare system. Gene therapy and cannabinoid-based therapies have recently gained popularity, with promising outcomes for treating Dravet Syndrome. This mirrors a larger trend toward individualized treatment, which addresses the specific requirements of South Korean patients. Opportunities are growing as the South Korean pharmaceutical industry prioritizes research and development for uncommon illnesses. Collaborations between academic institutions and biotech businesses promote innovation and allow for the investigation of novel treatment approaches. Furthermore,
South Korea's favorable regulatory framework for clinical trials creates a unique opportunity for the discovery and approval of innovative medications that especially target Dravet Syndrome. This collaborative mentality may complement the emphasis on patient access, ensuring that innovative medicines reach impacted families quickly. Recent trends also show an increased emphasis on support networks for families afflicted with Dravet syndrome. Initiatives to raise awareness and education about the illness are becoming increasingly common, improving knowledge of this complicated ailment in both the medical profession and the general population. Furthermore, the proliferation of internet platforms facilitates relationships between families, caregivers, and healthcare professionals, opening the door for shared experiences and collective advocacy. This comprehensive strategy strives to enhance the quality of life for patients and families affected by Dravet Syndrome in South Korea.
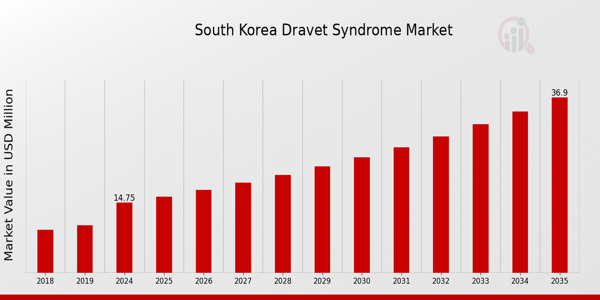
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
South Korea Dravet Syndrome Market Drivers
Rising Prevalence of Dravet Syndrome
The South Korea Dravet Syndrome Market Industry is experiencing a significant upsurge due to the increasing prevalence of Dravet Syndrome, a rare but severe form of epilepsy in children. According to the Centers for Disease Control and Prevention (CDC), the incidence of pediatric epilepsy disorders, including Dravet syndrome, is estimated to be approximately 7.6 per 1,000 children in South Korea. This rising number translates to more diagnosed cases annually, establishing a strong market for effective treatment options.The government of South Korea has been focusing heavily on advancing healthcare policies and support frameworks to cater to this patient population, indicating a societal commitment to improving healthcare solutions for epilepsy, including Dravet syndrome.
Additionally, the Korean Epilepsy Society emphasizes the need for expanding awareness and better treatment protocols, which aligns with the market's growth trajectory in the country. With approximately 1 in 15,000 births leading to Dravet Syndrome, the forecast suggests an increased market potential to meet the medicinal needs of these patients across South Korea.
Innovative Treatment Development
The South Korea Dravet Syndrome Market Industry is buoyed by the rapid development of innovative treatment options aimed specifically at Dravet syndrome. With the emergence of novel therapies and pharmaceuticals like cannabinoids and gene therapy, there is a promising increase in the available treatment landscape. The Ministry of Food and Drug Safety in South Korea has been facilitating pathways for expedited drug approvals, especially for rare diseases.For example, Epidiolex, a cannabidiol product, has shown significant efficacy in reducing seizures in Dravet syndrome patients. This regulatory support aims to streamline the introduction of effective drugs into the market, nurturing a competitive environment for pharmaceutical companies to invest more in research and development targeted towards Dravet syndrome. Recent statistics revealed that the presence of new drug approvals can raise market growth by nearly 40%, demonstrating how innovation can significantly impact patient care and market dynamics.
Increasing Awareness and Education Programs
Awareness and education surrounding Dravet syndrome are increasing, which is positively influencing the South Korea Dravet Syndrome Market Industry. Non-profit organizations and medical associations in South Korea, such as the Korean Society of Child Neurology, are actively conducting seminars, workshops, and campaigns to educate both healthcare practitioners and the general public about Dravet syndrome, its symptoms, and available treatment options.A significant outcome of these efforts indicates that misdiagnosis rates have decreased by approximately 30%, allowing for timely intervention and treatment. These initiatives are crucial since early diagnosis can substantially improve outcomes for children suffering from Dravet syndrome. As a result, the market has witnessed sharper demand for treatment solutions, leading to an estimated increase in market potential by 15% as more families pursue professional medical advice upon recognizing symptoms.
South Korea Dravet Syndrome Market Segment Insights:
Dravet Syndrome Market Type of Seizures Insights
The South Korea Dravet Syndrome Market is characterized by its diverse range of seizure types, each exhibiting unique clinical features and implications for treatment strategies. Myoclonic seizures, which involve brief, involuntary muscle jerks, are particularly important due to their frequent occurrence in individuals with Dravet syndrome, often exacerbating the overall seizure burden. Atonic seizures, characterized by sudden loss of muscle tone, can lead to significant safety concerns and require careful management strategies to prevent injuries.Partial seizures, which may present as focal onset seizures, contribute to a complex profile of seizure activity that can affect diagnosis and treatment.
Absence seizures, though less common in Dravet syndrome, may still be relevant, as they require differentiation from other types of seizures in clinical evaluation. Tonic seizures, recognizable by sudden stiffness or contraction of the muscles, are significant contributors to the overall seizure frequency in patients. Additionally, photosensitive seizures triggered by visual stimuli highlight the necessity for individualized management strategies tailored to specific seizure types, considering that such triggers can vary widely among patients.Lastly, the categorization of 'Others' encompasses a range of seizure presentations that don't fit neatly into the defined types, illustrating the heterogeneity of Dravet syndrome and the importance of comprehensive care approaches. As the market evolves, understanding these types of seizures is critical for developing more effective therapeutic interventions suited to individual patient needs, thereby improving overall patient outcomes in South Korea's growing healthcare landscape. The comprehensive approach in the South Korea Dravet Syndrome Market ensures that treatment plans are reflective of each seizure type's significance, fostering better management and enhancing the quality of life for affected individuals.
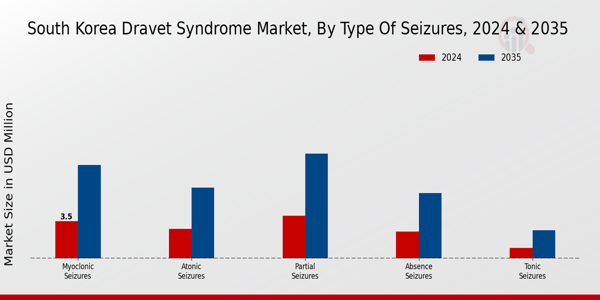
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Dravet Syndrome Market Disease Diagnosis Insights
The South Korea Dravet Syndrome Market, particularly focusing on the Disease Diagnosis segment, plays a crucial role in identifying and managing this severe epilepsy form. Magnetic Resonance Imaging (MRI) is significant as it provides detailed brain images, helping clinicians understand structural abnormalities associated with Dravet Syndrome. Electroencephalography (EEG) is another vital tool, enabling real-time monitoring of electrical brain activity, essential for diagnosing seizures characteristic of this condition. SCN1A testing is particularly important, as mutations in the SCN1A gene are common in Dravet Syndrome, facilitating genetic diagnosis and informing treatment options.
Other diagnostic methods contribute to comprehensive evaluation but are often supplemental to these primary techniques. The growing prevalence of Dravet Syndrome awareness in South Korea is driving market growth, supported by advancements in technology and research. Furthermore, challenges such as the need for training healthcare professionals on these diagnostic tools emphasize the importance of ongoing education in optimizing diagnosis and care.
Dravet Syndrome Market Treatment Management Insights
The Treatment Management segment of the South Korea Dravet Syndrome Market remains crucial in addressing the needs of patients suffering from this severe form of epilepsy. This segment includes various approaches, such as Seizure Medications, which play a significant role in controlling and reducing the frequency of seizures experienced by patients, underscoring their dominant presence in the market. The Ketogenic Diet, recognized for its therapeutic effects, has become an essential management strategy for pediatric patients, often utilized in conjunction with medications for enhanced effectiveness.
Vagus Nerve Stimulation is another promising avenue that features innovative technology to alleviate seizures, appealing to patients seeking alternative treatments when conventional medications fail. Additionally, alternative options are explored under 'Others,' which reflect the ongoing pursuit of effective management strategies in the market. With the increase in awareness and support from healthcare professionals in South Korea, the Treatment Management segment stands to benefit from improvements in access to care and innovative treatment solutions tailored to the unique needs of Dravet syndrome patients.The ongoing research and development in this segment underscore the potential for significant therapeutic advancements in the coming years.
Dravet Syndrome Market End-User Insights
The South Korea Dravet Syndrome Market is significantly shaped by its diverse End-User landscape, which includes Pharmaceutical Companies, Hospitals, Diagnostic Laboratories, Academic Research Institutes, and others. Pharmaceutical Companies play a crucial role, focusing on drug development and innovation tailored for Dravet Syndrome, contributing significantly to the overall market's growth. Hospitals serve as key infrastructures for diagnosis and management of the syndrome, emphasizing the need for specialized treatment facilities. Diagnostic Laboratories provide essential services in identifying Dravet Syndrome through advanced testing, enabling timely intervention, while Academic Research Institutes are pivotal in furthering understanding and treatment options for the condition.The synergy between these entities fosters a robust ecosystem that enhances patient care and research advancement. The South Korean government actively supports medical advancements, ensuring a conducive environment for these organizations to thrive, ultimately benefiting patients with Dravet Syndrome. The collaborative nature of these End-Users positions the South Korea Dravet Syndrome Market to address the complex challenges posed by this rare epilepsy syndrome effectively.
South Korea Dravet Syndrome Market Key Players and Competitive Insights:
The competitive landscape of the South Korea Dravet Syndrome Market has been characterized by a growing focus on innovative treatment options for this severe genetic epilepsy. With an increasing awareness of Dravet syndrome among healthcare professionals and patients, the market has experienced significant growth due to the rising incidence of the condition and the demand for effective therapies. The competitive dynamics are influenced by various factors, including advancements in drug development, regulatory approvals, and partnerships between pharmaceutical companies and research institutions.
Companies are striving to leverage their research capabilities and establish a strong market presence by delivering safe and effective treatment solutions that alleviate the challenges faced by those affected by the condition. This dynamic marketplace is witnessing increased investments in research and development activities and collaborations, paving the way for potential breakthroughs in the treatment of Dravet syndrome in South Korea.Medicenna Therapeutics has emerged as a notable player within the South Korea Dravet Syndrome Market, primarily due to its innovative approach to immunotherapy and targeted treatments.
The company focuses on developing therapies that enhance the effectiveness of existing treatments, giving it a distinctive edge in the competitive landscape. Medicenna Therapeutics leverages its advanced research capabilities to formulate novel therapeutics that address the specific needs of Dravet syndrome patients. With a commitment to facilitating better patient outcomes, the company is concentrating on building strong relationships with healthcare practitioners and local stakeholders, thereby enhancing its market footprint in South Korea.
Its strengths lie in its robust portfolio of investigational products designed to combat severe epilepsy syndromes, solidifying its position as a key player in this specialized domain.UCB has established a prominent presence in the South Korean Dravet Syndrome Market with its focus on developing therapeutic solutions that address challenging neurological conditions. The company is well-recognized for its leading product offerings, specifically tailored to manage Dravet syndrome and improve the quality of life for patients. UCB's strength lies in its extensive research pipeline and commitment to continuous innovation, which has enabled it to introduce effective treatments into the market.
The company maintains strong collaborations with healthcare institutions and research organizations, fostering a collaborative approach to advancing treatment modalities. Furthermore, UCB has made strategic mergers and acquisitions that bolster its capabilities within the neurology sector, ensuring a competitive edge in the dynamic South Korean market. By focusing on patient-centric solutions and actively participating in educational initiatives, UCB is well-positioned to address the unmet needs of Dravet syndrome patients in South Korea.
Key Companies in the South Korea Dravet Syndrome Market Include:
- Medicenna Therapeutics
- UCB
- Novartis
- Ovid Therapeutics
- Biogen
- Pfizer
- Eisai
- Sarepta Therapeutics
- NeuroRx
- Spark Therapeutics
- Zogenix
- GW Pharmaceuticals
- Acorda Therapeutics
- Jazz Pharmaceuticals
- Marinus Pharmaceuticals
South Korea Dravet Syndrome Market Industry Developments
The South Korea Dravet Syndrome market is experiencing notable advancements, with ongoing developments from key players like UCB, Novartis, and GW Pharmaceuticals focusing on innovative therapies and treatments. In recent months, Medicenna Therapeutics announced progress in its ongoing clinical trials aimed at evaluating its novel therapies for epilepsy, contributing to the broader landscape of treatment options for Dravet Syndrome. Additionally, Zogenix's leading product, Fintepla, has shown promising results in mitigating episodes and is gaining acceptance among healthcare providers in South Korea. The market outlook remains positive as the South Korean government emphasizes rare disease treatments, enhancing research grants and regulatory support for these companies. Notably, Pfizer's collaboration with local biotech firms signals a strategic commitment to integrating advanced therapies into the market. Market valuation for the Dravet Syndrome segment has seen growth, attributed to increased awareness and diagnosis rates within healthcare systems. Significant historical developments, including FDA approvals in late 2022 for treatments aimed specifically at Dravet Syndrome, continue to influence market dynamics, underscoring the urgency for comprehensive patient support and innovative solutions in South Korea.
South Korea Dravet Syndrome Market Segmentation Insights
South Korea Dravet Syndrome MarketType of SeizuresOutlook
- Myoclonic seizures
- Atonic seizures
- Partial seizures
- Absence seizures
- Tonic seizures
- Photosensitive seizures
- Others
South Korea Dravet Syndrome MarketDisease DiagnosisOutlook
- Magnetic Resonance Imaging
- Electroencephalography
- SCN1A testing
- Others
South Korea Dravet Syndrome MarketTreatment ManagementOutlook
South Korea Dravet Syndrome MarketEnd-UserOutlook
- Pharmaceutical Companies
- Hospitals
- Diagnostic Laboratories
- Academic research institutes
- Others
| Report Attribute/Metric Source: |
Details |
| MARKET SIZE 2018 |
13.75(USD Million) |
| MARKET SIZE 2024 |
14.75(USD Million) |
| MARKET SIZE 2035 |
36.9(USD Million) |
| COMPOUND ANNUAL GROWTH RATE (CAGR) |
8.693% (2025 - 2035) |
| REPORT COVERAGE |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| BASE YEAR |
2024 |
| MARKET FORECAST PERIOD |
2025 - 2035 |
| HISTORICAL DATA |
2019 - 2024 |
| MARKET FORECAST UNITS |
USD Million |
| KEY COMPANIES PROFILED |
Medicenna Therapeutics, UCB, Novartis, Ovid Therapeutics, Biogen, Pfizer, Eisai, Sarepta Therapeutics, NeuroRx, Spark Therapeutics, Zogenix, GW Pharmaceuticals, Acorda Therapeutics, Jazz Pharmaceuticals, Marinus Pharmaceuticals |
| SEGMENTS COVERED |
Type of Seizures, Disease Diagnosis, Treatment & Management, End-User |
| KEY MARKET OPPORTUNITIES |
Increased prevalence awareness initiatives, Innovative treatment development opportunities, Expansion of genetic testing services, Government funding for research, Enhanced patient support programs |
| KEY MARKET DYNAMICS |
Increasing prevalence of Dravet Syndrome, Growing awareness and diagnosis, Advancements in treatment options, Strong research and development activities, Government support and funding |
| COUNTRIES COVERED |
South Korea |
Frequently Asked Questions (FAQ) :
The South Korea Dravet Syndrome Market is expected to be valued at 14.75 million USD in 2024.
By 2035, the South Korea Dravet Syndrome Market is projected to reach a value of 36.9 million USD.
The expected CAGR for the South Korea Dravet Syndrome Market from 2025 to 2035 is 8.693%.
Myoclonic seizures are expected to dominate the market, valued at 8.75 million USD in 2035.
The market value for partial seizures is projected to be 9.8 million USD by 2035.
Key players in the market include Medicenna Therapeutics, UCB, Novartis, and Biogen among others.
The expected market value for atonic seizures in 2024 is 2.75 million USD.
Absence seizures are projected to reach a market value of 6.1 million USD by 2035.
Growth opportunities in the market are driven by rising awareness and advancements in treatment options.
Challenges may include regulatory hurdles and the high cost of innovative therapies.
















