-
Definition
-
Scope Of The Study
- Research Objective
- Limitations
-
Primary Research
-
Secondary Research
-
Market
-
Drivers
-
Restraints
-
Opportunities
-
Challenges
-
Macroeconomic Indicators
- Bargaining Power Of Suppliers
- Bargaining Power Of Buyers
- Threat Of New Entrants
- Intensity Of Rivalry
-
Value Chain Analysis
-
Investment Feasibility Analysis
-
Pricing Analysis
-
Global Sex Cord–Gonadal Stromal Tumor Market, By Tumor Type
-
Introduction
-
Granulosa Cell Tumor
- Market Estimates & Forecast, 2020–2027
-
Sertoli Cell Tumor
- Market Estimates & Forecast, 2020–2027
-
Thecoma
- Market Estimates & Forecast, 2020–2027
- Market Estimates & Forecast, 2020–2027
-
6.5
-
Leydig Cell Tumor
-
Sertoli-Leydig Cell Tumor
- Market Estimates & Forecast, 2020–2027
-
Gynandroblastoma
- Market Estimates & Forecast, 2020–2027
-
Sex Cord Tumor With Annular Tubules (SCTAT)
- Market Estimates &
-
Forecast, 2020–2027
-
Chapter 7. Global Sex Cord–Gonadal Stromal
-
Tumor Market, By Diagnosis
-
Introduction
-
Microscopy
-
7.2.1
-
Market Estimates & Forecast, 2020–2027
-
Immunohistochemistry
- Market Estimates & Forecast, 2020–2027
-
Tumor Marker
- Market Estimates & Forecast, 2020–2027
-
Ultrasound
- Market Estimates & Forecast, 2020–2027
-
MRI
-
7.6.1
-
Market Estimates & Forecast, 2020–2027
-
Others
- Market
-
Estimates & Forecast, 2020–2027
-
Chapter 8. Global Sex Cord–Gonadal
-
Stromal Tumor Market, By Treatment
-
Introduction
-
Chemotherapy
- Market Estimates & Forecast, 2020–2027
-
Radiotherapy
- Market Estimates & Forecast, 2020–2027
-
Surgery
-
8.4.1
-
Market Estimates & Forecast, 2020–2027
-
Others
- Market
-
Estimates & Forecast, 2020–2027
-
Chapter 9. Global Sex Cord–Gonadal
-
Stromal Tumor Market, By End-User
-
Introduction
-
Hospitals &
- Market Estimates & Forecast, 2020–2027
-
Clinics
-
Cancer
- Market Estimates & Forecast, 2020–2027
-
Research Centers
-
Research And Academic Institutes
- Market Estimates & Forecast,
-
Others
- Market Estimates & Forecast, 2020–2027
-
Chapter 12. Global Sex Cord–Gonadal Stromal Tumor Market, By Region
-
12.1
-
Introduction
-
Americas
- North America
- South America
-
Europe
- Western
- Eastern
-
Europe
-
12.3.1.4
-
Spain
-
Europe
-
Asia Pacific
- Japan
- China
- India
- Australia
- Republic Of Korea
- Rest Of Asia Pacific
-
The Middle East & Africa
- United Arab Emirates
- Oman
- Kuwait
- Qatar
- Rest
-
12.5.2
-
Saudi Arabia
-
Of The Middle East & Africa
-
Chapter 13 Company Landscape
-
Introduction
-
Market Share Analysis
-
Key Development & Strategies
-
13.3.1
-
Key Developments
-
Chapter 14 Company Profiles
-
Abbott
- Company
- Tumor Types Overview
- Financials
- SWOT
-
Overview
-
Analysis
-
Beckman, Dickinson And Company (BD)
- Company Overview
- Tumor Types Overview
- Financial Overview
- Key Developments
- SWOT Analysis
-
Johnson & Johnson Services, Inc.
- Tumor Types Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
14.3.1
-
Company Overview
-
Bio-Rad Laboratories,
- Company Overview
- Tumor Types/Business Segment Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
Inc.
-
BOSTON SCIENTIFIC CORPORATION
- Company Overview
- Tumor
- Financial Overview
- Key Developments
- Company Overview
- Tumor Types Overview
- Financial Overview
- Key Developments
-
Types Overview
-
14.6
-
CooperSurgical Inc.
-
DANAHER CORPORATION
- Overview
- Tumor Types Overview
- Financials
- SWOT Analysis
-
14.7.4
-
Key Developments
-
Others
-
Chapter 15 MRFR
-
Conclusion
-
Key Findings
- From CEO’s View Point
-
15.1.2
-
Unmet Needs Of The Market
-
Key Companies To Watch
-
Prediction
-
Of The Sex Cord–Gonadal Stromal Tumor Industry
-
Chapter 16 Appendix
-
LIST OF TABLES
-
Sex Cord–Gonadal Stromal Tumor Industry Synopsis,
-
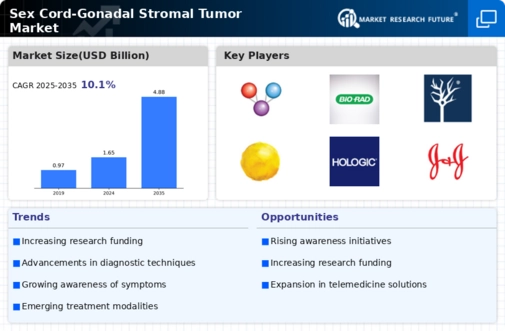
Sex Cord–Gonadal Stromal Tumor Market Estimates
-
& Forecast, 2020–2027, (USD Million)
-
Sex Cord–Gonadal
-
Stromal Tumor Market, By Region, 2020–2027, (USD Million)
-
Sex
-
Cord–Gonadal Stromal Tumor Market, By Tumor Type, 2020–2027, (USD Million)
-
Sex Cord–Gonadal Stromal Tumor Market, By Diagnosis, 2020–2027,
-
(USD Million)
-
Sex Cord–Gonadal Stromal Tumor Market, By Treatment,
-
Sex Cord–Gonadal Stromal Tumor
-
Market, By End-User, 2020–2027, (USD Million)
-
North America Sex
-
Cord–Gonadal Stromal Tumor Market, By Tumor Type, 2020–2027, (USD Million)
-
North America Sex Cord–Gonadal Stromal Tumor Market, By Diagnosis,
-
North America Sex Cord–Gonadal
-
Stromal Tumor Market, By Treatment, 2020–2027, (USD Million)
-
Table 11
-
North America Sex Cord–Gonadal Stromal Tumor Market, By End-User, 2020–2027,
-
(USD Million)
-
U.S. Sex Cord–Gonadal Stromal Tumor Market, By
-
Tumor Type, 2020–2027, (USD Million)
-
U.S. Sex Cord–Gonadal
-
Stromal Tumor Market, By Diagnosis, 2020–2027, (USD Million)
-
Table 14
-
U.S. Sex Cord–Gonadal Stromal Tumor Market, By Treatment, 2020–2027,
-
(USD Million)
-
U.S. Sex Cord–Gonadal Stromal Tumor Market, By
-
End-User, 2020–2027, (USD Million)
-
Canada Sex Cord–Gonadal
-
Stromal Tumor Market, By Tumor Type, 2020–2027, (USD Million)
-
Table 17
-
Canada Sex Cord–Gonadal Stromal Tumor Market, By Diagnosis, 2020–2027,
-
(USD Million)
-
Canada Sex Cord–Gonadal Stromal Tumor Market,
-
By Treatment, 2020–2027, (USD Million)
-
Canada Sex Cord–Gonadal
-
Stromal Tumor Market, By End-User, 2020–2027, (USD Million)
-
Table 20
-
South America Sex Cord–Gonadal Stromal Tumor Market, By Tumor Type, 2020–2027,
-
(USD Million)
-
South America Sex Cord–Gonadal Stromal Tumor Market,
-
By Diagnosis, 2020–2027, (USD Million)
-
South America Sex Cord–Gonadal
-
Stromal Tumor Market, By Treatment, 2020–2027, (USD Million)
-
Table 23
-
South America Sex Cord–Gonadal Stromal Tumor Market, By End-User, 2020–2027,
-
(USD Million)
-
Europe Sex Cord–Gonadal Stromal Tumor Market,
-
By Tumor Type, 2020–2027, (USD Million)
-
Europe Sex Cord–Gonadal
-
Stromal Tumor Market, By Diagnosis, 2020–2027, (USD Million)
-
Table 26
-
Europe Sex Cord–Gonadal Stromal Tumor Market, By Treatment, 2020–2027,
-
(USD Million)
-
Europe Sex Cord–Gonadal Stromal Tumor Market,
-
By End-User, 2020–2027, (USD Million)
-
Western Europe Sex Cord–Gonadal
-
Stromal Tumor Market, By Tumor Type, 2020–2027, (USD Million)
-
Table 29
-
Western Europe Sex Cord–Gonadal Stromal Tumor Market, By Diagnosis, 2020–2027,
-
(USD Million)
-
Western Europe Sex Cord–Gonadal Stromal Tumor
-
Market, By Treatment, 2020–2027, (USD Million)
-
Western Europe
-
Sex Cord–Gonadal Stromal Tumor Market, By End-User, 2020–2027, (USD
-
Million)
-
Eastern Europe Sex Cord–Gonadal Stromal Tumor Market,
-
By Tumor Type, 2020–2027, (USD Million)
-
Eastern Europe Sex Cord–Gonadal
-
Stromal Tumor Market, By Diagnosis, 2020–2027, (USD Million)
-
Table 34
-
Eastern Europe Sex Cord–Gonadal Stromal Tumor Market, By Treatment, 2020–2027,
-
(USD Million)
-
Eastern Europe Sex Cord–Gonadal Stromal Tumor
-
Market, By End-User, 2020–2027, (USD Million)
-
Asia Pacific Sex
-
Cord–Gonadal Stromal Tumor Market, By Tumor Type, 2020–2027, (USD Million)
-
Asia Pacific Sex Cord–Gonadal Stromal Tumor Market, By Diagnosis,
-
Asia Pacific Sex Cord–Gonadal
-
Stromal Tumor Market, By Treatment, 2020–2027, (USD Million)
-
Table 39
-
Asia Pacific Sex Cord–Gonadal Stromal Tumor Market, By End-User, 2020–2027,
-
(USD Million)
-
The Middle East & Africa Sex Cord–Gonadal
-
Stromal Tumor Market, By Tumor Type, 2020–2027, (USD Million)
-
Table 41
-
The Middle East & Africa Sex Cord–Gonadal Stromal Tumor Market, By Diagnosis,
-
The Middle East & Africa Sex Cord–Gonadal
-
Stromal Tumor Market, By Treatment, 2020–2027, (USD Million)
-
Table 43
-
The Middle East & Africa Sex Cord–Gonadal Stromal Tumor Market, By End-User,
-
LIST OF FIGURES
-
Research Process
-
Segmentation For Sex Cord–Gonadal Stromal Tumor Market
-
Figure
-
Segmentation Market Dynamics For Sex Cord–Gonadal Stromal Tumor Market
-
Global Sex Cord–Gonadal Stromal Tumor Market Share, By Tumor Type
-
Global Sex Cord–Gonadal Stromal Tumor Market Share, By
-
Diagnosis 2020
-
Global Sex Cord–Gonadal Stromal Tumor Market
-
Share, By Treatment 2020
-
Global Sex Cord–Gonadal Stromal Tumor
-
Market Share, By End-User 2020
-
Global Sex Cord–Gonadal Stromal
-
Tumor Market Share, By Region, 2020
-
North America Sex Cord–Gonadal
-
Stromal Tumor Market Share, By Country, 2020
-
Europe Sex Cord–Gonadal
-
Stromal Tumor Market Share, By Country, 2020
-
Asia Pacific Sex Cord–Gonadal
-
Stromal Tumor Market Share, By Country, 2020
-
Middle East & Africa
-
Sex Cord–Gonadal Stromal Tumor Market Share, By Country, 2020
-
Figure
-
Global Sex Cord–Gonadal Stromal Tumor Market: Company Share Analysis, 2020
-
(%)
-
Abbott: Key Financials
-
Abbott: Segmental Revenue
-
Abbott: Geographical Revenue
-
Beckman, Dickinson And Company
-
(BD): Key Financials
-
Beckman, Dickinson And Company (BD): Segmental
-
Revenue
-
Beckman, Dickinson And Company (BD): Geographical Revenue
-
Johnson & Johnson Services, Inc.: Key Financials
-
Figure 21
-
Johnson & Johnson Services, Inc.: Segmental Revenue
-
Johnson &
-
Johnson Services, Inc.: Geographical Revenue
-
Bio-Rad Laboratories,
-
Inc.: Key Financials
-
Bio-Rad Laboratories, Inc.: Segmental Revenue
-
Bio-Rad Laboratories, Inc.: Geographical Revenue
-
BOSTON
-
SCIENTIFIC CORPORATION: Key Financials
-
BOSTON SCIENTIFIC CORPORATION:
-
Segmental Revenue
-
BOSTON SCIENTIFIC CORPORATION Geographical Revenue
-
CooperSurgical Inc.: Key Financials
-
CooperSurgical Inc.:
-
Segmental Revenue
-
CooperSurgical Inc.: Geographical Revenue
-
Figure
-
DANAHER CORPORATION: Key Financials
-
DANAHER CORPORATION: Segmental
-
Revenue
-
DANAHER CORPORATION: Geographical Revenue












Leave a Comment