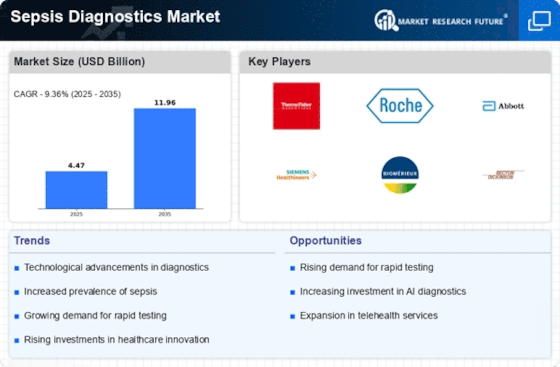
Top Industry Leaders in the Sepsis Diagnostics Market

Latest Sepsis Diagnostics Companies Update
December 2023: Legend Biotech receives China National Medical Products Administration (NMPA) approval for LCAR-017 for relapsed/refractory B-cell lymphomas. This signifies the first regulatory approval for CAR T-cell therapy in China.November 2023: BioMarker has raised $30 million in a Series B funding round, demonstrating investor confidence in the company's AI-powered platform for analyzing brain scans and improving diagnosis of neurological disorders. Jan 2024: BlueRock Therapeutics releases encouraging Phase I/II data for autologous T-cell therapy for PD-L1 negative non-small cell lung cancer. this marks progress in tackling challenging cancer types with autologous therapies.October 2023: CTI BioTech's blood test for early sepsis detection, SepsiSTAT, wins FDA Breakthrough Device Designation. This could expedite the approval process and bring the test to market faster.Jan 2024: Magenta Therapeutics secures $165 million in Series D funding, aiming to advance product development and manufacturing capabilities.August 2023: Novartis expands CAR-T program partnership with Cellares, demonstrating continued commitment to CAR T-cell therapy development.
-
Sandstone Diagnostics Inc. (US)
-
T2 Biosystems
-
Immunexpress (US)
-
Axis-Shield Diagnostics Ltd. (Scotland)
-
Becton Dickinson and Company (US)
-
EKF Diagnostics (UK)
-
Cube Dx GmbH (Austria)
-
Wolters Kulwer N.V. (Netherlands)
-
Amara Health (US)