Increase in Aging Population
The Retinal Vein Occlusion Market is experiencing a notable increase in demand due to the aging population. As individuals age, the risk of developing retinal disorders, including retinal vein occlusion, escalates significantly. Statistics indicate that the prevalence of retinal vein occlusion is higher in older adults, with estimates suggesting that approximately 1.6 million individuals in the United States alone are affected. This demographic shift is likely to drive the market as healthcare providers seek effective treatment options for this vulnerable population. Furthermore, the aging population often presents with comorbidities such as hypertension and diabetes, which are known risk factors for retinal vein occlusion. Consequently, the Retinal Vein Occlusion Market is poised for growth as healthcare systems adapt to meet the needs of an increasingly elderly demographic.
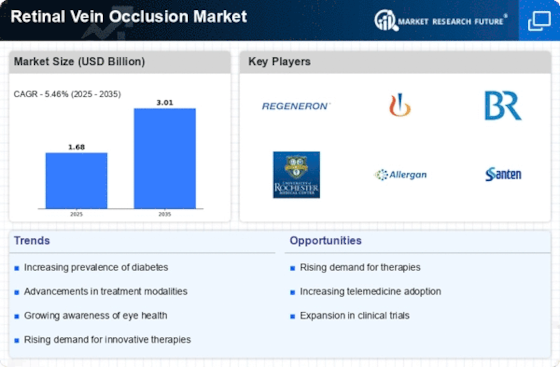
Increase in Diabetes Prevalence
The rising prevalence of diabetes is a substantial driver for the Retinal Vein Occlusion Market. Diabetes is a well-established risk factor for retinal vein occlusion, with studies indicating that individuals with diabetes are at a higher risk of developing this condition. Current estimates suggest that over 400 million people worldwide are living with diabetes, and this number is projected to increase in the coming years. As the diabetic population grows, so does the incidence of diabetic retinopathy and retinal vein occlusion, leading to heightened demand for effective treatment options. Healthcare systems are increasingly focusing on managing diabetic complications, which includes addressing retinal disorders. Consequently, the Retinal Vein Occlusion Market is expected to expand as healthcare providers seek to develop targeted therapies and interventions for this at-risk population.
Technological Innovations in Treatment
Technological advancements are playing a pivotal role in shaping the Retinal Vein Occlusion Market. Innovations in treatment modalities, such as the development of anti-VEGF (vascular endothelial growth factor) therapies, have revolutionized the management of retinal vein occlusion. These therapies have demonstrated efficacy in reducing macular edema and improving visual outcomes, leading to increased adoption among healthcare providers. Market data suggests that the anti-VEGF segment is expected to witness substantial growth, driven by ongoing clinical trials and the introduction of new formulations. Additionally, advancements in diagnostic technologies, including optical coherence tomography (OCT), are enhancing the ability to detect and monitor retinal vein occlusion, thereby facilitating timely intervention. As these technologies continue to evolve, the Retinal Vein Occlusion Market is likely to expand, offering patients improved treatment options and outcomes.
Rising Awareness and Screening Programs
The Retinal Vein Occlusion Market is benefiting from increased awareness and the implementation of screening programs aimed at early detection of retinal disorders. Public health initiatives and educational campaigns are effectively informing individuals about the risk factors and symptoms associated with retinal vein occlusion. This heightened awareness is encouraging more patients to seek regular eye examinations, leading to earlier diagnosis and treatment. Market data indicates that regions with robust screening programs have reported a higher incidence of diagnosed cases, which in turn drives demand for therapeutic interventions. Furthermore, healthcare providers are increasingly recognizing the importance of proactive screening, particularly for at-risk populations such as those with diabetes or hypertension. As awareness continues to grow, the Retinal Vein Occlusion Market is expected to see a corresponding increase in patient engagement and treatment uptake.
Growing Investment in Research and Development
Investment in research and development is a critical driver for the Retinal Vein Occlusion Market. Pharmaceutical companies and research institutions are increasingly allocating resources to explore novel treatment options and improve existing therapies for retinal vein occlusion. This trend is evidenced by the rising number of clinical trials focused on innovative drug formulations and combination therapies. Market data reveals that R&D spending in ophthalmology has surged, reflecting a commitment to addressing unmet medical needs in retinal disorders. Additionally, collaborations between academia and industry are fostering the development of cutting-edge technologies, such as gene therapy and regenerative medicine, which hold promise for the future of retinal vein occlusion treatment. As these investments yield new therapeutic options, the Retinal Vein Occlusion Market is likely to experience significant growth and transformation.

















Leave a Comment