Market Analysis
In-depth Analysis of Relapsing Remitting Multiple Sclerosis Market Industry Landscape
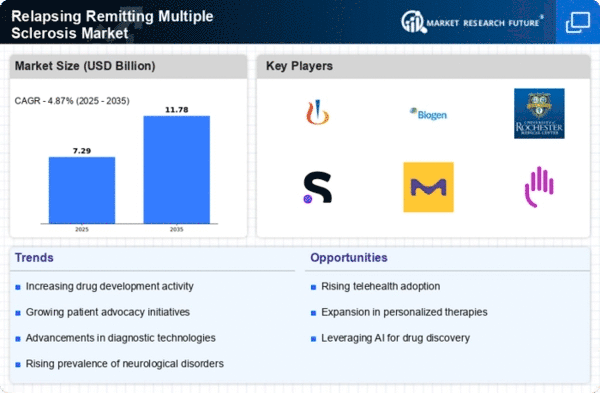
The global market is mainly driven by the growing prevalence of multiple sclerosis (especially its RR form). With higher numbers diagnosed with Relapsing–remitting multiple sclerosis (RRMS) characterized by periods of remission followed by episodes during which symptoms worsen; the need for advanced targeted treatments increases.
Market dynamics are significantly influenced by continuous innovations in approaches to treating RRMS. Pharmaceutical corporations together with scientists are involved into creation of disease modifying therapies (DMTs) aimed at decreasing frequency/ severity of relapses; delaying disease progression; improving quality of life of patients with RRMS. The treatment landscape has been broadened by the introduction of innovative DMTs, including monoclonal antibodies and oral medications giving a wider range of options for dealing with different forms of the disease.
In addition to this, collaboration between neurologists, patient advocacy groups and pharmaceutical companies is one of the driving forces behind RRMS treatment. Comprehensive care for RRMS individuals is ensured by multidisciplinary approaches that include healthcare professionals from a number of areas. Collaborations also go into research and development where partnerships enhance discovery of new therapeutic targets and development of new treatment modes.
Market forces including Healthcare spending and insurance coverage have significant impact on market dynamics. More so, appropriate reimbursement for treatments for RRMS implies that healthcare providers are more likely to adopt advanced costlier drugs thereby encouraging market growth. On another note, governments and insurers are forced to invest in innovative treatments that can lower societal costs as well as economic burden associated with managing MS such as long-term disability costs and rehabilitation.
However, grave concerns still exist in the RRMS treatment market. For instance, some advanced therapies are too costly while others don’t work as expected in all individuals. In addition, the high prices of innovative DMTs and managing RRMS pose obstacles to their dissemination; this is especially evident in areas with limited means for providing healthcare.

















Leave a Comment