Market Analysis
In-depth Analysis of Recombinant DNA Technology Market Industry Landscape
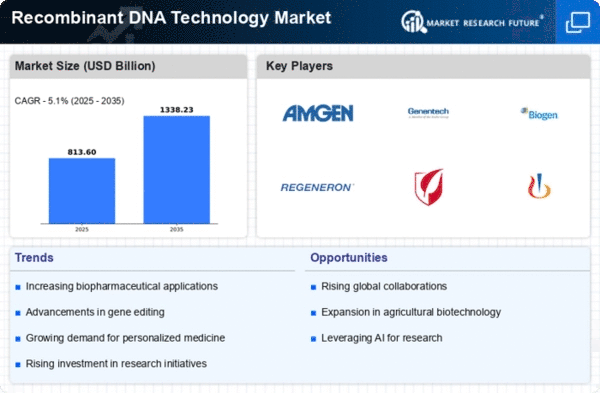
Because of new technologies, the need for breakthrough treatments, and precise medicine, the market for recombinant DNA technology is moving very quickly. By mixing and changing genetic material, this method makes hybrid DNA molecules. It is possible to make biopharmaceuticals, genetically edited animals, and tests. Recombinant DNA biopharmaceuticals revolutionize the market. This approach may produce genetically engineered yeast or bacteria that produce proteins, hormones, and antibodies that cure many diseases. The market is changing because of the growing focus on custom medicines. Personalized treatments are possible with recombinant DNA technology because it lets drugs be made to fit the genetic background of each patient. This method is becoming more popular in cancer treatment and other healing areas, which changes how people act. A big part of gene therapy's fast growth is recombinant DNA technology. Changing genes and putting them into specific cells could help treat genetic problems, cancer, and other illnesses. Gene therapy improvements are what make the market grow. Recombinant DNA is used to help make medicines. Scientists can use this method to make organisms that show antigens from bacteria or viruses. This makes vaccines safer and more effective. The way the market works is affected by the creation of vaccines around the world, especially for new dangerous illnesses. In agriculture, recombinant DNA technology is often used to make GMOs with features that farmers want. Agricultural engineering and food security are affected by crops that are better able to handle pests, diseases, and changes in the environment. Rules about modified DNA technologies change how the market works. R&D and marketing are both affected by strict rules about the safety and morals of genetically edited products. Genetically modified organism ethics change the way markets are set up. CRISPR-Cas9 is a new tool for modified DNA technology that makes progress. CRISPR-Cas9 has changed genetic engineering and medical study by allowing precise and focused editing of genomes. Gene editing and medicine are made possible by CRISPR-Cas9 and recombinant DNA tools. The recombinant DNA business is shaped by the work of biotechnology, research, and medicine companies. These connections help with new ideas, getting resources, and sharing knowledge, which makes markets bigger. Companies that want to get a bigger part of the market are very competitive. To move recombinant DNA technology forward, both old and new biotechnology companies fight through study, product sales, and smart relationships. As people learn more about genetic engineering and biopharmaceuticals and healthcare systems get better, recombinant DNA technology is growing in developing countries. These markets are affected by new biotechnologies, rules and regulations, and the cost of goods.

















Leave a Comment