Rising Incidence of Syphilis
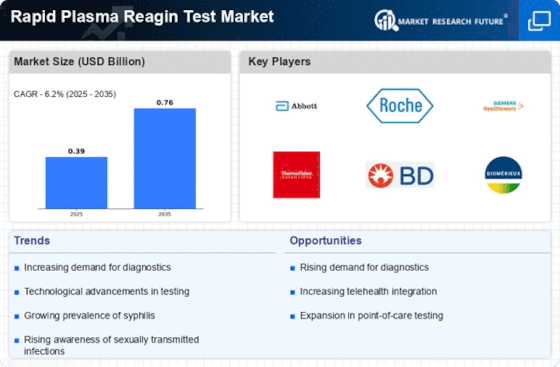
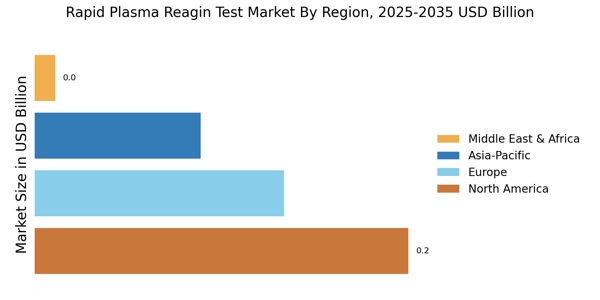
The rising incidence of syphilis and other sexually transmitted infections (STIs) appears to be a primary driver for the Rapid Plasma Reagin Test Market. According to recent data, syphilis rates have shown a concerning upward trend, particularly among certain demographics. This increase necessitates effective screening and diagnostic tools, thereby enhancing the demand for rapid plasma reagin tests. The ability of these tests to provide quick results is particularly appealing in clinical settings where timely diagnosis is crucial. As healthcare providers seek to address this public health challenge, the market for rapid plasma reagin tests is likely to expand, reflecting the urgent need for effective STI management.
Government Initiatives and Funding
Government initiatives aimed at improving public health outcomes significantly influence the Rapid Plasma Reagin Test Market. Various health departments have launched campaigns to promote STI testing and treatment, often accompanied by funding for diagnostic tools. For instance, increased budget allocations for sexual health programs have been observed, which directly impacts the availability and accessibility of rapid plasma reagin tests. These initiatives not only raise awareness but also facilitate the integration of these tests into routine healthcare practices. Consequently, the market is expected to experience growth as more healthcare facilities adopt these tests to comply with public health guidelines.
Growing Demand for Rapid Diagnostics
The growing demand for rapid diagnostics in healthcare settings is a notable driver for the Rapid Plasma Reagin Test Market. Healthcare providers increasingly prioritize tests that yield quick results, enabling timely treatment decisions. This trend is particularly evident in emergency departments and outpatient clinics, where the need for immediate diagnosis is paramount. The rapid plasma reagin test, known for its efficiency and reliability, aligns well with this demand. Market data indicates that the rapid diagnostics segment is projected to witness substantial growth, further propelling the adoption of rapid plasma reagin tests as a preferred option for syphilis screening.
Increased Focus on Preventive Healthcare
An increased focus on preventive healthcare is shaping the landscape of the Rapid Plasma Reagin Test Market. As healthcare systems worldwide shift towards preventive measures, the importance of early detection of STIs, including syphilis, has gained prominence. Rapid plasma reagin tests serve as a vital tool in this preventive approach, allowing for early diagnosis and treatment. This shift is supported by various health organizations advocating for routine screening, particularly in high-risk populations. The market is likely to benefit from this trend, as healthcare providers incorporate these tests into their preventive health strategies, thereby enhancing overall public health outcomes.
Technological Innovations in Testing Methods
Technological innovations in testing methods are driving advancements in the Rapid Plasma Reagin Test Market. The introduction of automated systems and enhanced testing protocols has improved the accuracy and efficiency of rapid plasma reagin tests. These innovations not only streamline the testing process but also reduce the likelihood of false positives and negatives, which is crucial for effective patient management. As laboratories and healthcare facilities adopt these advanced technologies, the market is expected to expand. Furthermore, the integration of digital health solutions, such as telemedicine, may facilitate the distribution and utilization of rapid plasma reagin tests, further enhancing their market presence.