Market Trends
Key Emerging Trends in the Pharmaceutical Quality Control Market
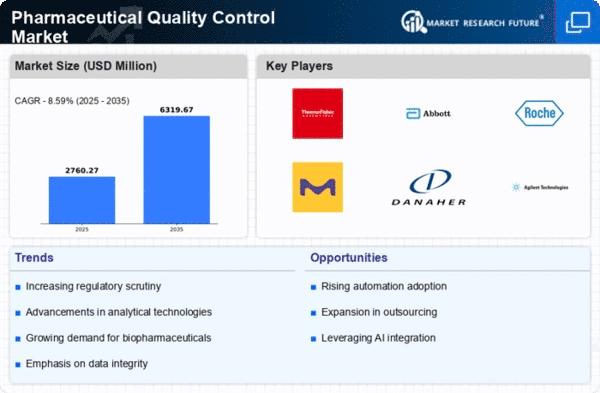
The pharmaceutical quality control market is growing fast because the worldwide medicine business wants to make sure that drugs are safe and work well. As rules get stricter, strong checks are needed more and more to control quality. Groups like the FDA and EMA are making their rules stronger. They tell pharmaceutical companies they must follow tough quality checks for good reason. This made businesses spend money on new tools and ways to follow changing rules set by the government. The market is changing towards complex analysis tools like chromatography, spectroscopy and mass spectrometry. These tools allow for accurate and dependable study of medicine products, making sure they follow rules. As medicines get more complicated because of improvements in drug delivery and biotechnology, the need for advanced quality control has gotten stronger. Businesses are using new ways to measure the quality of complicated mixtures correctly. A lot of pharmaceutical companies are sending their quality checks to experts in the field. This way of doing things is pushed by the need to cut down expenses for running a business, get special know-how and make it easier to manage. Companies that do research are very important in giving quality control services. Making sure data is correct and keeping full records have become very important in checking the quality of medicines. There is a growing interest in using electronic systems and computer programs that make data more accurate, traceable, and comply with rules. Old ways to test tiny living things take too long. This slows down when new medicines can be sent out. The market is seeing a move towards fast microbe testing methods that give results quickly without losing accuracy. This is very important for making sure drugs get to the market quickly. The world-wide pharmaceutical supply chains have added problems with keeping high quality in different places across the globe. Businesses are using the same safety rules to make sure that product quality is consistent no matter where it's made. In the pharmaceutical industry, machines like AI and IoT along with Big Data are being added more often to check quality. These tools make it easier to automate, look at data and make decisions. They help quality control become more effective and efficient too.

















Leave a Comment