-
EXECUTIVE SUMMARY
-
Market Attractiveness Analysis
- Pediatric Medical Device Market, by Product
- Pediatric Medical Device Market, by End User
- Pediatric Medical Device Market, by Region
-
MARKET INTRODUCTION
-
Definition
-
Scope of the Study
- Research Objective
- Assumptions
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
-
Breakdown of Primary Respondents
-
Forecasting Techniques
-
Research Methodology for Market Size Estimation
-
Bottom-Up Approach
-
Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Porter’s Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
Value Chain Analysis
- R&D and Designing
- Manufacturing
- Distribution & Sales
- Post Sales Services
-
Pediatric Medical Device Market, BY PRODUCT
-
Overview
-
Cardiology Devices
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
In-Vitro Diagnostic (IVD) Devices
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Diagnostic Imaging Devices
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Telemedicine
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Anesthesia and Respiratory Care Devices
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Neonatal ICU Devices
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Monitoring Devices
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Others
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Pediatric Medical Device Market, BY END USER
-
Overview
-
Hospitals
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Pediatric Clinics
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Ambulatory Surgery Centers
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Research and Academic Institutes
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Others
-
Market Estimates & Pediatric Medical Device Market, by Region, 2023-2030
-
Market Estimates & Pediatric Medical Device Market, by Country, 2023-2030
-
Pediatric Medical Device Market, BY REGION
-
Overview
-
Americas
- North America
- Latin America
-
Europe
- Western Europe
- Eastern Europe
-
Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
-
Middle East & Africa
- Middle East
- Africa
-
COMPANY LANDSCAPE
-
Overview
-
Competitor Dashboard
-
Major Growth Strategies in the Pediatric Medical Devices Market
-
Competitive Benchmarking
-
The Leading Players in terms of Number of Developments in the Pediatric Medical Devices Market
-
Key Developments & Growth Strategies
-
Major Market Players Financial Matrix & Market Ratio
-
COMPANY PROFILES
-
Abbott Laboratories (US)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Boston Scientific Corporation (US)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Medtronic PLC (Ireland)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Cardinal Health (US)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
F. Hoffmann-La Roche Ltd (Switzerland)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
GE Healthcare (US)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Philips Healthcare (US)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Baxter (US)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Siemens Healthineers (Germany)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
Stryker Corporation (US)
- Company Overview
- Products/Services Offered
- Financial Overview
- Key Developments
- SWOT Analysis
- Key Strategies
-
APPENDIX
-
References
-
Related Reports
-
-
LIST OF TABLES
-
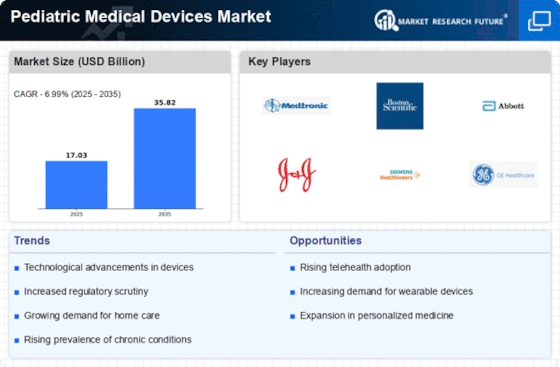
GLOBAL PEDIATRIC MEDICAL DEVICES MARKET SYNOPSIS, 2023-2030
-
GLOBAL PEDIATRIC MEDICAL DEVICES MARKET ESTIMATES & FORECAST, 2023-2030 (USD MILLION)
-
Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
Pediatric Medical Device Market, BY REGION, 2023-2030 (USD MILLION)
-
NORTH AMERICA: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
NORTH AMERICA: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
US: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
US: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
CANADA: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
CANADA: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
LATIN AMERICA: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
LATIN AMERICA: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
EUROPE: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
EUROPE: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
WESTERN EUROPE: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
WESTERN EUROPE: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
EASTERN EUROPE: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
EASTERN EUROPE: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
ASIA-PACIFIC: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
ASIA-PACIFIC: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
MIDDLE EAST & AFRICA: Pediatric Medical Device Market, BY PRODUCT, 2023-2030 (USD MILLION)
-
MIDDLE EAST & AFRICA: Pediatric Medical Device Market, BY END USER, 2023-2030 (USD MILLION)
-
-
LIST OF FIGURES
-
RESEARCH PROCESS
-
MARKET STRUCTURE OF THE GLOBAL PEDIATRIC MEDICAL DEVICES MARKET
-
MARKET DYNAMICS OF THE GLOBAL PEDIATRIC MEDICAL DEVICES MARKET
-
Pediatric Medical Device Market, BY PRODUCT, 2020 (%)
-
Pediatric Medical Device Market, BY END USER, 2020 (%)
-
Pediatric Medical Device Market, BY REGION, 2020 (%)
-
AMERICAS: PEDIATRIC MEDICAL DEVICES MARKET SHARE BY REGION, 2020 (%)
-
NORTH AMERICA: Pediatric Medical Device Market, BY COUNTRY, 2020 (%)
-
EUROPE: Pediatric Medical Device Market, BY REGION, 2020 (%)
-
WESTERN EUROPE: Pediatric Medical Device Market, BY COUNTRY, 2020 (%)
-
ASIA-PACIFIC: Pediatric Medical Device Market, BY COUNTRY, 2020 (%)
-
MIDDLE EAST & AFRICA: Pediatric Medical Device Market, BY COUNTRY, 2020 (%)
-
GLOBAL PEDIATRIC MEDICAL DEVICES MARKET: COMPANY SHARE ANALYSIS, 2020 (%)
-
ABBOTT LABORATORIES: KEY FINANCIALS
-
ABBOTT LABORATORIES: SEGMENTAL REVENUE
-
ABBOTT LABORATORIES: REGIONAL REVENUE
-
BOSTON SCIENTIFIC CORPORATION: KEY FINANCIALS
-
BOSTON SCIENTIFIC CORPORATION: SEGMENTAL REVENUE
-
BOSTON SCIENTIFIC CORPORATION: REGIONAL REVENUE
-
MEDTRONIC PLC: KEY FINANCIALS
-
MEDTRONIC PLC: SEGMENTAL REVENUE
-
MEDTRONIC PLC: REGIONAL REVENUE
-
CARDINAL HEALTH: KEY FINANCIALS
-
CARDINAL HEALTH: SEGMENTAL REVENUE
-
CARDINAL HEALTH: REGIONAL REVENUE
-
F. HOFFMANN-LA ROCHE LTD: KEY FINANCIALS
-
F. HOFFMANN-LA ROCHE LTD: SEGMENTAL REVENUE
-
F. HOFFMANN-LA ROCHE LTD: REGIONAL REVENUE
-
GE HEALTHCARE: KEY FINANCIALS
-
GE HEALTHCARE: SEGMENTAL REVENUE
-
GE HEALTHCARE: REGIONAL REVENUE
-
PHILIPS HEALTHCARE: KEY FINANCIALS
-
PHILIPS HEALTHCARE: SEGMENTAL REVENUE
-
PHILIPS HEALTHCARE: REGIONAL REVENUE
-
BAXTER: KEY FINANCIALS
-
BAXTER: SEGMENTAL REVENUE
-
BAXTER: REGIONAL REVENUE
-
SIEMENS HEALTHINEERS: KEY FINANCIALS
-
SIEMENS HEALTHINEERS: SEGMENTAL REVENUE
-
SIEMENS HEALTHINEERS: REGIONAL REVENUE
-
STRYKER CORPORATION: KEY FINANCIALS
-
STRYKER CORPORATION: SEGMENTAL REVENUE
-
STRYKER CORPORATION: REGIONAL REVENUE
















Leave a Comment