Market Trends
Key Emerging Trends in the Orphan Drugs Market
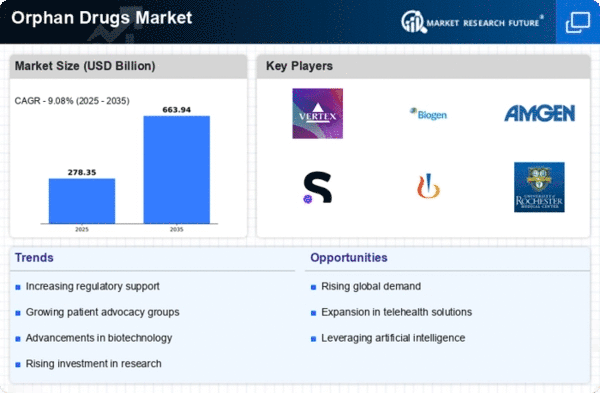
A rare disease, also known as an orphan disease, is a medical condition that affects only a small percentage of the population. These diseases are often genetic in nature, meaning they are present throughout a person's entire life, even if symptoms do not appear immediately. The global prevalence of rare diseases is on the rise, with 6,084 rare diseases reported in 2016, according to a survey by the QuintilesIMS Institute. While some rare conditions may affect only a handful of individuals, others, like cystic fibrosis, impact hundreds or thousands of people, with approximately 30,000 individuals affected in the United States alone. Collectively, rare disease patients make up about 6% to 8% of the population.
Several factors contribute to the increasing prevalence of rare diseases, including the growing trend of delayed parenthood and increased use of fertility therapies. As a result, there is a broad diversity of approximately 6,000 rare diseases, each characterized by unique disorders. The symptoms not only vary from disease to disease but can also differ among patients with the same condition. This variability in symptoms can lead to misdiagnosis and delays in receiving proper treatment.
The causes of rare diseases are diverse, with the majority being genetic in origin, directly resulting from changes in genes or chromosomes. The rarity and complexity of these diseases pose challenges for accurate diagnosis and effective treatment. Additionally, the limited understanding of many rare diseases further complicates the development of targeted therapies.
It is crucial to address the unique challenges posed by rare diseases, including the need for specialized medical care, research, and awareness. Advances in genetic testing and research technologies are providing new opportunities to better understand and diagnose rare diseases. Collaborative efforts among healthcare professionals, researchers, and patient advocacy groups are essential in improving the lives of individuals affected by rare diseases. Increased awareness, research funding, and a focus on innovative therapies are key components in addressing the unique needs of this population and working towards better outcomes for those living with rare diseases.

















Leave a Comment