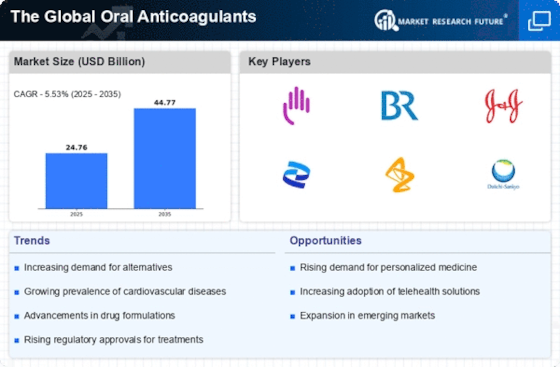
Top Industry Leaders in the Oral Anticoagulants Market
 Latest oral anticoagulant Companies Update
Latest oral anticoagulant Companies Update
-
May 2023: Asundexian, an experimental medication developed by Bayer for the treatment of atrial fibrillation (AF), was awarded Fast Track Designation by the U.S. Food and medication Administration (FDA). This comes after asundexian received its first U.S. FDA Fast Track Designation in 2022 for the treatment of individuals who have had a non-cardioembolic ischemic stroke. Part of the larger Phase III OCEANIC clinical trial program, which has included over 27,000 patients in over 40 countries, is the OCEANIC-AF (atrial fibrillation) study with asundexian. With the goal of decoupling efficacy from increased bleeding risk, asundexian is being studied as a potential superior therapeutic option in stroke prevention and might be part of a new class of treatments in thrombosis management.
-
September 2022: Anthos Therapeutics, a clinical-stage biotechnology company developing innovative cardiovascular and metabolic disease treatments, announced that the FDA has granted Fast Track designation for the investigation of abelacimab for the prevention of stroke and systemic embolism in atrial fibrillation patients. In July 2022, the FDA also granted abelacimab for cancer-associated thrombosis (CAT) Fast Track status. This is Anthos' second certification in two months. Abelacimab began Phase 3 studies this year as the first Factor XI inhibitor. Factor XI inhibitors may separate thrombosis and normal clotting processes. This new family of anticoagulants should be equally effective as existing therapies and safer. Patients who struggle with everyday pill swallowing will benefit from alternate delivery and less frequent doses.
List of oral anticoagulant Key companies in the market
- AstraZeneca Plc (UK)
- Pfizer (US)
- Boehringer Ingelheim GmbH (Germany)
- Daiichi Sankyo Company Ltd (Japan)
- Abbott Laboratories (US)
- Eli Lilly & Company (US)
- GlaxoSmithKline Plc (UK)
- Portola Pharmaceuticals Inc (US)
- Medicure (Canada)
- Johnson & Johnson Services Inc (US)